The m.3243A>G mtDNA mutation is pathogenic in an in vitro model of the human blood brain barrier
- PMID: 19682606
- PMCID: PMC2783492
- DOI: 10.1016/j.mito.2009.08.006
The m.3243A>G mtDNA mutation is pathogenic in an in vitro model of the human blood brain barrier
Abstract
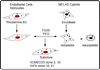
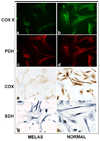
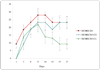
MELAS is a common mitochondrial disease frequently associated with the m.3243A>G point mutation in the tRNA(Leu(UUR)) of mitochondrial DNA and characterized by stroke-like episodes with vasogenic edema and lactic acidosis. The pathogenic mechanism of stroke and brain edema is not known. Alterations in the blood brain barrier (BBB) caused by respiratory chain defects in the cortical microvessels could explain the pathogenesis. To test this hypothesis we developed a tissue culture model of the human BBB. The MELAS mutation was introduced into immortalized brain capillary endothelial cells and astrocytes. Respiratory chain activity and transendothelial electrical resistance, TEER was measured. Severe defects of respiratory chain complex I and IV activities, and a moderate deficiency of complex II activity in cells harboring the MELAS mutation were associated with low TEER, indicating that the integrity of the BBB was compromised. These data support our hypothesis that respiratory chain defects in the components of the BBB cause changes in permeability.
Figures



Similar articles
-
Metabolically induced heteroplasmy shifting and l-arginine treatment reduce the energetic defect in a neuronal-like model of MELAS.Biochim Biophys Acta. 2012 Jun;1822(6):1019-29. doi: 10.1016/j.bbadis.2012.01.010. Epub 2012 Jan 28. Biochim Biophys Acta. 2012. PMID: 22306605 Free PMC article.
-
Accumulation of mtDNA with a mutation at position 3271 in tRNA(Leu)(UUR) gene introduced from a MELAS patient to HeLa cells lacking mtDNA results in progressive inhibition of mitochondrial respiratory function.Biochem Biophys Res Commun. 1993 Dec 30;197(3):1049-55. doi: 10.1006/bbrc.1993.2584. Biochem Biophys Res Commun. 1993. PMID: 8280119
-
Heteroplasmy and phenotype spectrum of the mitochondrial tRNALeu (UUR) gene m.3243A>G mutation in seven Han Chinese families.J Neurol Sci. 2020 Jan 15;408:116562. doi: 10.1016/j.jns.2019.116562. Epub 2019 Nov 6. J Neurol Sci. 2020. PMID: 31722256
-
[MELAS (mitochondrial myopathy, encephalopathy lactic acidosis, and stroke-like episodes): clinical features and mitochondrial DNA mutations].Nihon Rinsho. 1993 Sep;51(9):2373-8. Nihon Rinsho. 1993. PMID: 8411715 Review. Japanese.
-
MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options.Mol Genet Metab. 2015 Sep-Oct;116(1-2):4-12. doi: 10.1016/j.ymgme.2015.06.004. Epub 2015 Jun 15. Mol Genet Metab. 2015. PMID: 26095523 Review.
Cited by
-
Quantitative measurement of cerebral oxygen extraction fraction using MRI in patients with MELAS.PLoS One. 2013 Nov 8;8(11):e79859. doi: 10.1371/journal.pone.0079859. eCollection 2013. PLoS One. 2013. PMID: 24260310 Free PMC article.
-
Metabolically induced heteroplasmy shifting and l-arginine treatment reduce the energetic defect in a neuronal-like model of MELAS.Biochim Biophys Acta. 2012 Jun;1822(6):1019-29. doi: 10.1016/j.bbadis.2012.01.010. Epub 2012 Jan 28. Biochim Biophys Acta. 2012. PMID: 22306605 Free PMC article.
-
Drug Penetration into the Central Nervous System: Pharmacokinetic Concepts and In Vitro Model Systems.Pharmaceutics. 2021 Sep 23;13(10):1542. doi: 10.3390/pharmaceutics13101542. Pharmaceutics. 2021. PMID: 34683835 Free PMC article. Review.
-
Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Episodes (MELAS): A Case Report and Critical Reappraisal of Treatment Options.Pediatr Neurol. 2016 Mar;56:59-61. doi: 10.1016/j.pediatrneurol.2015.12.010. Epub 2015 Dec 19. Pediatr Neurol. 2016. PMID: 26797286 Free PMC article.
-
Metabolic myopathies.Curr Rheumatol Rep. 2010 Oct;12(5):386-93. doi: 10.1007/s11926-010-0119-9. Curr Rheumatol Rep. 2010. PMID: 20676808
References
-
- Betts J, Jaros E, Perry RH, Schaefer AM, Taylor RW, et al. Molecular neuropathology of MELAS: Level of heteroplasmy in individual neurones and evidence of extensive vascular involvement. Neuropathol. Appl. Neurobiol. 2006;32:359–373. - PubMed
-
- Bonilla E, Sciacco M, Tanji K, Sparaco M, Petruzzella V, et al. New morphological approaches to the study of mitochondrial encephalomyopathies. Brain. Pathol. 1992;2:113–119. - PubMed
-
- Davidson MM, Nesti C, Palenzuela L, Walker WF, Hernandez E, et al. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005;39:133–147. - PubMed
-
- Finsterer J. Genetic, pathogenetic, and phenotypic implications of the mitochondrial A3243G trnaleu(uur) mutation. Acta Neurol. Scand. 2007;116:1–14. - PubMed
-
- Hasegawa H, Matsuoka T, Nonaka Y GotoI. Strongly succinate dehydrogenase-reactive blood vessels in muscles from patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. Ann. Neurol. 1991;29:601–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

