Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers
- PMID: 19683342
- PMCID: PMC2745602
- DOI: 10.1016/j.biomaterials.2009.07.063
Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers
Abstract
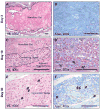
Biomaterials that promote angiogenesis have great potential in regenerative medicine for rapid revascularization of damaged tissue, survival of transplanted cells, and healing of chronic wounds. Supramolecular nanofibers formed by self-assembly of a heparin-binding peptide amphiphile and heparan sulfate-like glycosaminoglycans were evaluated here using a dorsal skinfold chamber model to dynamically monitor the interaction between the nanofiber gel and the microcirculation, representing a novel application of this model. We paired this model with a conventional subcutaneous implantation model for static histological assessment of the interactions between the gel and host tissue. In the static analysis, the heparan sulfate-containing nanofiber gels were found to persist in the tissue for up to 30 days and revealed excellent biocompatibility. Strikingly, as the nanofiber gel biodegraded, we observed the formation of a de novo vascularized connective tissue. In the dynamic experiments using the dorsal skinfold chamber, the material again demonstrated good biocompatibility, with minimal dilation of the microcirculation and only a few adherent leukocytes, monitored through intravital fluorescence microscopy. The new application of the dorsal skinfold model corroborated our findings from the traditional static histology, demonstrating the potential use of this technique to dynamically evaluate the biocompatibility of materials. The observed biocompatibility and development of new vascularized tissue using both techniques demonstrates the potential of these angiogenesis-promoting materials for a host of regenerative strategies.
Figures


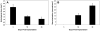




References
-
- Stupp SI. Biomaterials for regenerative medicine. Mrs Bulletin. 2005;30(7):546–553.
-
- Stupp SI, Donners JJJM, Li LS, Mata A. Expanding frontiers in biomaterials. Mrs Bulletin. 2005;30(11):864–873.
-
- Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. - PubMed
-
- Velichko YS, Stupp SI, de la Cruz MO. Molecular simulation study of peptide amphiphile self-assembly. J Phys Chem B. 2008:2326–2334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

