Gut commensal bacteria direct a protective immune response against Toxoplasma gondii
- PMID: 19683684
- PMCID: PMC2746820
- DOI: 10.1016/j.chom.2009.06.005
Gut commensal bacteria direct a protective immune response against Toxoplasma gondii
Abstract
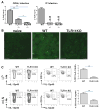
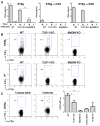
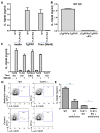
Toxoplasma gondii is a universally distributed pathogen that infects over one billion people worldwide. Host resistance to this protozoan parasite depends on a Th1 immune response with potent production of the cytokines interleukin-12 and interferon gamma. Although Toll-like receptor 11 (TLR11) plays a major role in controlling Th1 immunity to this pathogen in mice, this innate immune receptor is nonfunctional in humans, and the mechanisms of TLR11-independent sensing of T. gondii remain elusive. Here, we show that oral infection by T. gondii triggers a TLR11-independent but MyD88-dependent Th1 response that is impaired in TLR2xTLR4 double knockout and TLR9 single knockout mice. These mucosal innate and adaptive immune responses to T. gondii rely on the indirect stimulation of dendritic cells by normal gut microflora. Thus, our results reveal that gut commensal bacteria can serve as molecular adjuvants during parasitic infection, providing indirect immunostimulation that protects against T. gondii in the absence of TLR11.
Figures

Comment in
-
A gut feeling for microbes: getting it going between a parasite and its host.Cell Host Microbe. 2009 Aug 20;6(2):104-6. doi: 10.1016/j.chom.2009.07.009. Cell Host Microbe. 2009. PMID: 19683676 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

