Generation of primordial germ cells from pluripotent stem cells
- PMID: 19683852
- PMCID: PMC3847855
- DOI: 10.1016/j.diff.2009.07.001
Generation of primordial germ cells from pluripotent stem cells
Abstract
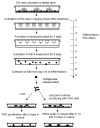
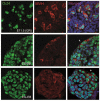
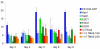
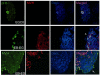
Embryonic stem (ES) cells, derived from pre-implantation embryo, embryonic germ (EG) cells, derived from embryonic precursors of gametes, primordial germ cells (PGCs), can differentiate into any cell type in the body. Moreover, ES cells have the capacity to differentiate into PGCs in vitro. In the present study we have shown the differentiation capacity of six EG cell lines to form PGCs in vitro, in comparison to ES cells. Cell lines were differentiated via embryoid body (EB) formation using the co-expression of mouse vasa homolog (Mvh) and Oct-4 to identify newly formed PGCs in vitro. We found an increase of PGC numbers in almost all analysed cell lines in 5-day-old EBs, thus suggesting that EG and ES cells have similar efficiency to generate PGCs. The addition of retinoic acid confirmed that the cultures had attained a PGC-like identity and continued to proliferate. Furthermore we have shown that the expression pattern of Prmt5 and H3K27me3 in newly formed PGCs is similar to that observed in embryonic day E11.5 PGCs in vivo. By co-culturing EBs with Chinese hamster ovary (CHO) cells some of the PGCs entered into meiosis, as judged by Scp3 expression. The derivation of germ cells from pluripotent stem cells in vitro could provide an invaluable model system to study both the genetic and epigenetic programming of germ cell development in vivo.
2009 International Society of Differentiation. Published by Elsevier Ltd
Figures




References
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–630. - PubMed
-
- Beddington RS, Robertson EJ. An assessment off the developmental potential of embryonic stem cells in the midgestation mouse embryos. Development. 1989;105:733–737. - PubMed
-
- De Felici M, Scaldaferri ML, Lobascio M, Iona S, Nazzicone V, Klinger FG, Farini D. Experimental approaches to the study of primordial germ cell lineage and proliferation. Hum Reprod Update. 2004;10(3):197–206. - PubMed
-
- Donovan PJ, De Miguel MP. Turning germ cells into stem cells. Curr Opin Genet Dev. 2003;13(5):463–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

