Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma
- PMID: 19683863
- PMCID: PMC2823929
- DOI: 10.1016/j.canlet.2009.07.016
Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma
Abstract
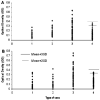
The identification and characterization of tumor-associated antigens (TAAs) and their use in antigen mini-arrays for cancer immunodiagnosis has been of interest recently as an approach to cancer detection. In this study, autoantibodies in sera from a patient with HCC were used as probes to immunoscreen a HepG2 cDNA expression library for the identification of TAAs involved in malignant liver transformation. Recombinant proteins from two genes identified in this manner, Sui1 and RalA were expressed, purified and used as antigens in immunoassays to detect the presence of antibodies in sera from 77 patients with HCC, 30 with chronic hepatitis (CH), 30 with liver cirrhosis (LC) and 82 normal human sera (NHS). The prevalence of antibody to Sui1 and RalA in HCC were 11.7% (9/77) and 19.5% (15/77), respectively, which were significantly higher than prevalence in liver cirrhosis (3.3% and 3.3%), chronic hepatitis (0% and 0%) and normal human sera (0% and 0%). When Sui1 and RalA were added to a panel of eight other TAAs used in a previous study, the final cumulative prevalence of anti-TAA antibodies in HCC to the 10 TAA array was raised to 66.2% (51/77). The specificity for HCC compared with LC, CH and NHS, was 66.7%, 80.0%, and 87.8%, respectively. When anti-TAA was added to abnormal serum AFP as combined diagnostic markers, it raised the diagnostic sensitivity from 66.2% to 88.7%. AFP and anti-TAA were independent markers and the simultaneous use of these two markers significantly resulted in the increased sensitivity of HCC detection.
Copyright 2009 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures

References
-
- Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71:26–35. - PubMed
-
- Covini G, Muhlen CAV, Pacchetti S, Colombo M, Chan EKL, Tan EM. Diversity of antinuclear antibody responses in hepatocellular carcinoma. J Hepatol. 1997;26:1255–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

