Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys
- PMID: 19684004
- PMCID: PMC2869048
- DOI: 10.1167/iovs.09-3847
Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys
Abstract
Purpose: AAV vectors produce stable transgene expression and elicit low immune response in many tissues. AAVs have been the vectors of choice for gene therapy for the eye, in particular the retina. scAAVs are modified AAVs that bypass the required second-strand DNA synthesis to achieve transcription of the transgene. The goal was to investigate the ability of AAV vectors to induce long-term, safe delivery of transgenes to the trabecular meshwork of living animals.
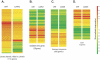
Methods: Single doses of AAV2.GFP and AAV2.RGD.GFP/Ad5.LacZ were injected intracamerally (IC) into rats (n = 28 eyes). A single dose of scAAV.GFP was IC-injected into rats (n = 72 eyes) and cynomolgus monkeys (n = 3). GFP expression was evaluated by fluorescence, immunohistochemistry, and noninvasive gonioscopy. Intraocular pressure (IOP) was measured with calibrated tonometer (rats) and Goldmann tonometer (monkeys). Differential expression of scAAV-infected human trabecular meshwork cells (HTM) was determined by microarrays. Humoral and cell-mediated immune responses were evaluated by ELISA and peripheral blood proliferation assays.
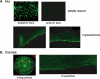
Results: No GFP transduction was observed on the anterior segment tissues of AAV-injected rats up to 27 days after injection. In contrast, scAAV2 transduced the trabecular meshwork very efficiently, with a fast onset (4 days). Eyes remained clear and no adverse effects were observed. Transgene expression lasted >3.5 months in rats and >2.35 years in monkeys.
Conclusions: The scAAV viral vector provides prolonged and safe transduction in the trabecular meshwork of rats and monkeys. The stable expression and safe properties of this vector could facilitate the development of trabecular meshwork drugs for gene therapy for glaucoma.
Figures





Similar articles
-
Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells.J Gene Med. 2006 May;8(5):589-602. doi: 10.1002/jgm.886. J Gene Med. 2006. PMID: 16506246
-
Capsid Mutated Adeno-Associated Virus Delivered to the Anterior Chamber Results in Efficient Transduction of Trabecular Meshwork in Mouse and Rat.PLoS One. 2015 Jun 8;10(6):e0128759. doi: 10.1371/journal.pone.0128759. eCollection 2015. PLoS One. 2015. PMID: 26052939 Free PMC article.
-
Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo.J Gene Med. 2001 Sep-Oct;3(5):437-49. doi: 10.1002/jgm.210. J Gene Med. 2001. PMID: 11601757
-
Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork.Exp Eye Res. 2015 Dec;141:33-41. doi: 10.1016/j.exer.2015.04.003. Epub 2015 May 27. Exp Eye Res. 2015. PMID: 26025608 Free PMC article. Review.
-
Gene transfer to the outflow tract.Exp Eye Res. 2017 May;158:73-84. doi: 10.1016/j.exer.2016.04.023. Epub 2016 Apr 27. Exp Eye Res. 2017. PMID: 27131906 Free PMC article. Review.
Cited by
-
Corneal gene therapy: basic science and translational perspective.Ocul Surf. 2013 Jul;11(3):150-64. doi: 10.1016/j.jtos.2012.10.004. Epub 2013 Feb 13. Ocul Surf. 2013. PMID: 23838017 Free PMC article. Review.
-
Lentiviral mediated delivery of CRISPR/Cas9 reduces intraocular pressure in a mouse model of myocilin glaucoma.Res Sq [Preprint]. 2023 Dec 19:rs.3.rs-3740880. doi: 10.21203/rs.3.rs-3740880/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 23;14(1):6958. doi: 10.1038/s41598-024-57286-6. PMID: 38196579 Free PMC article. Updated. Preprint.
-
AAV for Gene Therapy in Ocular Diseases: Progress and Prospects.Research (Wash D C). 2023 Dec 22;6:0291. doi: 10.34133/research.0291. eCollection 2023. Research (Wash D C). 2023. PMID: 38188726 Free PMC article. Review.
-
Evaluation of trabecular meshwork-specific promoters in vitro and in vivo using scAAV2 vectors expressing C3 transferase.Int J Ophthalmol. 2023 Aug 18;16(8):1196-1209. doi: 10.18240/ijo.2023.08.03. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37602341 Free PMC article.
-
The cellular and molecular biology of the iris, an overlooked tissue: the iris and pseudoexfoliation glaucoma.J Glaucoma. 2014 Oct-Nov;23(8 Suppl 1):S39-42. doi: 10.1097/IJG.0000000000000104. J Glaucoma. 2014. PMID: 25275904 Free PMC article. Review.
References
-
- Streilein JW. Immune Response and the Eye Boston: Karger; 1999
-
- Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci 1997; 38: 83–91 - PubMed
-
- Budenz DL, Bennett J, Alonso L, Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci 1995; 36: 2211–2215 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY013126/EY/NEI NIH HHS/United States
- P30 EY016665/EY/NEI NIH HHS/United States
- R01 EY011906/EY/NEI NIH HHS/United States
- EY02698/EY/NEI NIH HHS/United States
- HL051818/HL/NHLBI NIH HHS/United States
- EY11906/EY/NEI NIH HHS/United States
- R01 EY002698/EY/NEI NIH HHS/United States
- P01 HL051818/HL/NHLBI NIH HHS/United States
- 1R01EY019555-01/EY/NEI NIH HHS/United States
- KO8 NS045871-01/NS/NINDS NIH HHS/United States
- R01 EY019555/EY/NEI NIH HHS/United States
- K08 NS045871/NS/NINDS NIH HHS/United States
- EY13126/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

