Formation of amyloid fibrils in vitro from partially unfolded intermediates of human gammaC-crystallin
- PMID: 19684009
- PMCID: PMC2868461
- DOI: 10.1167/iovs.09-3987
Formation of amyloid fibrils in vitro from partially unfolded intermediates of human gammaC-crystallin
Abstract
Purpose: Mature-onset cataract results from the formation of light-scattering aggregates of lens crystallins. Although oxidative or mutational damage may be a prerequisite, little is known of the initiation or nucleation of these aggregated states. In mice carrying mutations in gamma-crystallin genes, a truncated form of gamma-crystallin formed intranuclear filamentous inclusions within lens fiber cells. Previous studies have shown that bovine crystallins and human gammaD-crystallin form amyloid fibrils under denaturing conditions in vitro. The amyloid fibril formation of human gammaC-crystallin (HgammaC-Crys) induced by low pH, together with characterization of a partially unfolded intermediate in the process were investigated.
Methods: HgammaC-Crys was expressed and purified from Escherichia coli. Partially unfolded intermediates were detected by tryptophan fluorescence spectroscopy and UV resonance Raman spectroscopy. The aggregation into amyloid fibrils was monitored by solution turbidity and fluorescence assay. The morphology of aggregates was characterized using transmission electron microscopy (TEM). Secondary structure of the peptides in their fibrillar state was characterized using Fourier transform infrared spectroscopy (FTIR).
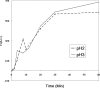
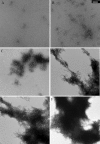
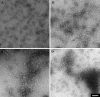
Results: The structure of HgammaC-Crys was perturbed at low pH. Partially unfolded intermediates were detected when solution pH was lowered to pH 3. At pH 3, HgammaC-Crys aggregated into amyloid fibrils. The kinetics and extent of the reaction was dependent on protein concentration, pH, and temperature. TEM images of aggregates revealed aggregation stages from short to long fibrils and from long fibrils to light-scattering fibril networks. FTIR spectroscopy confirmed the cross-beta character of the secondary structure of these fibrils.
Conclusions: HgammaC-Crys formed amyloid fibrils on incubation at low pH via a partially unfolded intermediate. This process could contribute to the early stages of the formation of light-scattering species in the eye lens.
Figures




Similar articles
-
Formation of amyloid fibrils in vitro by human gammaD-crystallin and its isolated domains.Mol Vis. 2008 Jan 16;14:81-9. Mol Vis. 2008. PMID: 18253099 Free PMC article.
-
Unravelling γD-crystallin aggregation pathway to understand cataract formation using fluorescence correlation spectroscopy.Mol Vis. 2025 May 17;31:190-202. eCollection 2025. Mol Vis. 2025. PMID: 40606473 Free PMC article.
-
Hydrophobic core mutations associated with cataract development in mice destabilize human gammaD-crystallin.J Biol Chem. 2009 Nov 27;284(48):33285-95. doi: 10.1074/jbc.M109.031344. Epub 2009 Sep 16. J Biol Chem. 2009. PMID: 19758984 Free PMC article.
-
The βγ-crystallins: native state stability and pathways to aggregation.Prog Biophys Mol Biol. 2014 Jul;115(1):32-41. doi: 10.1016/j.pbiomolbio.2014.05.002. Epub 2014 May 14. Prog Biophys Mol Biol. 2014. PMID: 24835736 Free PMC article. Review.
-
[Structure of amyloid fibrils].Pathologe. 2009 May;30(3):175-81. doi: 10.1007/s00292-009-1127-2. Pathologe. 2009. PMID: 19301007 Review. German.
Cited by
-
Trehalose Restrains the Fibril Load towards α-Lactalbumin Aggregation and Halts Fibrillation in a Concentration-Dependent Manner.Biomolecules. 2021 Mar 11;11(3):414. doi: 10.3390/biom11030414. Biomolecules. 2021. PMID: 33799517 Free PMC article.
-
Cataract-causing defect of a mutant γ-crystallin proceeds through an aggregation pathway which bypasses recognition by the α-crystallin chaperone.PLoS One. 2012;7(5):e37256. doi: 10.1371/journal.pone.0037256. Epub 2012 May 24. PLoS One. 2012. PMID: 22655036 Free PMC article.
-
Tyrosine/cysteine cluster sensitizing human γD-crystallin to ultraviolet radiation-induced photoaggregation in vitro.Biochemistry. 2014 Feb 18;53(6):979-90. doi: 10.1021/bi401397g. Epub 2014 Feb 5. Biochemistry. 2014. PMID: 24410332 Free PMC article.
-
Biophysical Elucidation of Fibrillation Inhibition by Sugar Osmolytes in α-Lactalbumin: Multispectroscopic and Molecular Docking Approaches.ACS Omega. 2020 Oct 8;5(41):26871-26882. doi: 10.1021/acsomega.0c04062. eCollection 2020 Oct 20. ACS Omega. 2020. PMID: 33111013 Free PMC article.
-
Characterization of a transient unfolding intermediate in a core mutant of γS-crystallin.J Mol Biol. 2011 Jan 21;405(3):840-50. doi: 10.1016/j.jmb.2010.11.005. Epub 2010 Nov 23. J Mol Biol. 2011. PMID: 21108948 Free PMC article.
References
-
- Harding JJ, Crabbe MJC. The lens: development, proteins, metabolism, and cataract. In: Dawson H. ed. The Eye London: Academic Press; 1984: 207–492
-
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res 2000; 71: 195–207 - PubMed
-
- Horwitz J, Emmons T, Takemoto L. The ability of lens alpha crystallin to protect against heat-induced aggregation is age-dependent. Curr Eye Res 1992; 11: 817–822 - PubMed
-
- Dobson CM. Protein folding and misfolding. Nature 2003; 426: 884–890 - PubMed
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 2004; 15: 3–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

