Radial spoke protein 3 is a mammalian protein kinase A-anchoring protein that binds ERK1/2
- PMID: 19684019
- PMCID: PMC2785576
- DOI: 10.1074/jbc.M109.048181
Radial spoke protein 3 is a mammalian protein kinase A-anchoring protein that binds ERK1/2
Abstract
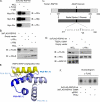
Initially identified in Chlamydomonas, RSP3 (radial spoke protein 3) is 1 of more than 20 identified radial spoke structural components of motile cilia and is required for axonemal sliding and flagellar motility. The mammalian orthologs for this and other radial spoke proteins, however, remain to be characterized. We found mammalian RSP3 to bind to the MAPK ERK2 through a yeast two-hybrid screen designed to identify interacting proteins that have a higher affinity for the phosphorylated, active form of the protein kinase. Consistent with the screening result, the human homolog, RSPH3, interacts with and is a substrate for ERK1/2. Moreover, RSPH3 is a protein kinase A-anchoring protein (AKAP) that scaffolds the cAMP-dependent protein kinase holoenzyme. The binding of RSPH3 to the regulatory subunits of cAMP-dependent protein kinase, RIIalpha and RIIbeta, is regulated by ERK1/2 activity and phosphorylation. Here we describe an ERK1/2-interacting AKAP and suggest a mechanism by which cAMP-dependent protein kinase-AKAP binding can be modulated by the activity of other enzymes.
Figures




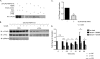
References
-
- Raman M., Chen W., Cobb M. H. (2007) Oncogene 26, 3100–3112 - PubMed
-
- Elion E. A. (2001) J. Cell Sci. 114, 3967–3978 - PubMed
-
- Kolch W. (2005) Nat. Rev. Mol. Cell Biol. 6, 827–837 - PubMed
-
- Morrison D. K., Davis R. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 91–118 - PubMed
-
- Rubin C. S. (1994) Biochim. Biophys. Acta 1224, 467–479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

