MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis
- PMID: 19684242
- PMCID: PMC2751950
- DOI: 10.1105/tpc.109.067819
MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis
Erratum in
- Plant Cell. 2010 Mar;22(3):991
Abstract
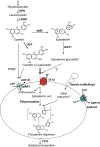
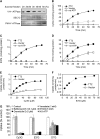
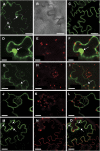
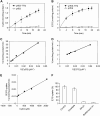
Expression of the Arabidopsis thaliana MYB transcription factor TRANSPARENT TESTA 2 (TT2) in Medicago trunculata hairy roots induces both proanthocyanidin accumulation and the ATP-dependent vacuolar/vesicular uptake of epicatechin 3'-O-glucoside; neither process is active in control roots that do, however, possess anthocyanidin 3-O-glucoside vacuolar uptake activity. A vacuolar membrane-localized multidrug and toxic compound extrusion (MATE) transporter, Medicago MATE1, was identified at the molecular level and shown to preferentially transport epicatechin 3'-O-glucoside. Genetic evidence has implicated TT12, a tonoplastic MATE transporter from Arabidopsis, in the transport of precursors for proanthocyanidin biosynthesis in the seed coat. However, although Arabidopsis TT12 facilitates the transport of cyanidin 3-O-glucoside into membrane vesicles when expressed in yeast, there is no evidence that cyanidin 3-O-glucoside is converted to proanthocyanidins after transport into the vacuole. Here, we show that Arabidopsis TT12, like Medicago MATE1, functions to transport epicatechin 3'-O-glucoside as a precursor for proanthocyanidin biosynthesis, and Medicago MATE1 complements the seed proanthocyanidin phenotype of the Arabidopsis tt12 mutant both quantitatively and qualitatively. On the basis of biochemical properties, tissue-specific expression pattern, and genetic loss-of-function analysis, we conclude that MATE1 is an essential membrane transporter for proanthocyanidin biosynthesis in the Medicago seed coat. Implications of these findings for the assembly of oligomeric proanthocyanidins are discussed.
Figures




References
-
- Abrahams, S., Lee, E., Walker, A.R., Tanner, G.J., Larkin, P., and Ashton, A.R. (2003). The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 35: 624–636. - PubMed
-
- Ariga, T., Asao, Y., Sugimoto, H., and Yokotuska, T. (1981). Occurrence of astringent oligomeric proanthocyanidins in legume seeds. Agric. Biol. Chem. 45: 2705–2708.
-
- Baxter, I.R., Young, J.C., Armstrong, G., Foster, N., Bogenschutz, N., Cordova, T., Peer, W.A., Hazen, S.P., Murphy, A.S., and Harper, J.F. (2005). A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102: 2649–2654. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

