Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells
- PMID: 19684584
- PMCID: PMC2782768
- DOI: 10.1038/nmat2515
Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells
Abstract
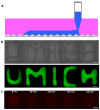
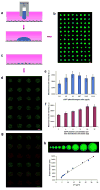
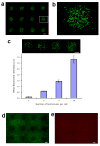
Microscale biopatterning enables regulation of cell-material interactions and cell shape, and enables multiplexed high-throughput studies in a cell- and reagent-efficient manner. The majority of available techniques rely on physical contact of a stamp, pin, or mask with mainly a dry surface. Inkjet and piezoelectric printing is carried out in a non-contact manner but still requires a substantially dry substrate to ensure fidelity of printed patterns. These existing methods, therefore, are limited for patterning onto delicate surfaces of living cells because physical contact or substantially dry conditions are damaging to them. Microfluidic patterning with laminar streams does enable non-contact patterning in fully aqueous environments but with limited throughput and reagent diffusion across interfacial flows. Here, we describe a polymeric aqueous two-phase system that enables patterning nanolitres of a reagent-containing aqueous phase, in arbitrary shapes, within a second aqueous phase covering a cell monolayer. With the appropriate medium formulation, reagents of interest remain confined to the patterned phase without significant diffusion. The fully aqueous environment ensures high reagent activity and cell viability. The utility of this strategy is demonstrated with patterned delivery of genetic materials to mammalian cells for phenotypic screening of gene expression and gene silencing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Patterning: Cells nourished by nanodrops.Nat Mater. 2009 Sep;8(9):700-2. doi: 10.1038/nmat2524. Nat Mater. 2009. PMID: 19701212 No abstract available.
References
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. - PubMed
-
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nature Biotech. 2008;26:120–126. - PubMed
-
- Singhvi R, et al. Engineering cell shape and function. Science. 1994;264:696–698. - PubMed
-
- Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotech. 2004;22:863–866. - PubMed
-
- Anderson DG, Putnam D, Lavik E, Mahmood T, Langer R. Biomaterial microarrays: Rapid, microscale screening of polymer-cell interaction. Biomater. 2005;26:4892–4897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

