The role of arginine-127 at the proximal NO-binding site in determining the electronic structure and function of 5-coordinate NO-heme in cytochrome c' of Rhodobacter sphaeroides
- PMID: 19685879
- PMCID: PMC3502046
- DOI: 10.1021/bi900833f
The role of arginine-127 at the proximal NO-binding site in determining the electronic structure and function of 5-coordinate NO-heme in cytochrome c' of Rhodobacter sphaeroides
Abstract
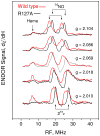
Cytochrome c' is a heme protein from a denitrifying variant of Rhodobacter sphaeroides which may serve to store and transport metabolic NO while protecting against NO toxicity. Its heme site bears resemblance through its 5-coordinate NO-binding capability to the regulatory site in soluble guanylate cyclase. A conserved arginine (Arg-127) abuts the 5-coordinate NO-heme binding site, and the alanine mutant R127A provided insight into the role of the Arg-127 in establishing the electronic structure of the heme-NO complex and in modifying the heme-centered redox potential and NO-binding affinity. By comparison to R127A, the wild-type Arg-127 was determined to increase the heme redox potential, diminish the NO-binding affinity, perturb and diminish the 14NO hyperfine coupling determined by ENDOR (electron nuclear double resonance), and increase the maximal electronic g-value. The larger isotropic NO hyperfine and the smaller maximal g-value of the R127A mutant together predicted that the Fe-N-O bond angle in the mutant is larger than that of the Arg-127-containing wild-type protein. Deuterium ENDOR provided evidence for exchangeable H/D consistent with hydrogen bonding of Arg-127, but not Ala-127, to the O of the NO. Proton ENDOR features previously assigned to Phe-14 on the distal side of the heme were unperturbed by the proximal side R127A mutation, implying the localized nature of that mutational perturbation at the proximal, NO-binding side of the heme. From this work two functions of positively charged Arg-127 emerged: the first was to maintain the KD of the cytochrome c' in the 1 microM range, and the second was to provide a redox potential that enhances the stability of the ferrous heme.
Figures









References
-
- Lawson DM, Stevenson CE, Andrew CR, George SJ, Eady RR. A two-faced molecule offers NO explanation: the proximal binding of nitric oxide to haem. Biochem Soc Trans. 2003;31:553–557. - PubMed
-
- Ramirez LM, Axelrod HL, Herron SR, Rupp B, Allen JP, Kantardjieff KA. High resolution crystal structure of ferricytochrome c′ from Rhodobacter sphaeroides. Journal of Chemical Crystallography. 2003;33:413–424.
-
- Choi PST. Ph D Thesis, Dept of Microbiology. Cornell; Ithaca, NY: 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

