Generation and biological activities of oxidized phospholipids
- PMID: 19686040
- PMCID: PMC3121779
- DOI: 10.1089/ars.2009.2597
Generation and biological activities of oxidized phospholipids
Abstract
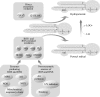
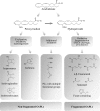
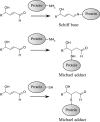
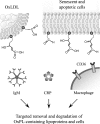
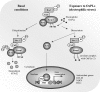
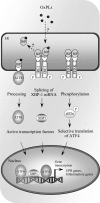
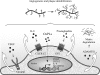
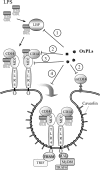
Glycerophospholipids represent a common class of lipids critically important for integrity of cellular membranes. Oxidation of esterified unsaturated fatty acids dramatically changes biological activities of phospholipids. Apart from impairment of their structural function, oxidation makes oxidized phospholipids (OxPLs) markers of "modified-self" type that are recognized by soluble and cell-associated receptors of innate immunity, including scavenger receptors, natural (germ line-encoded) antibodies, and C-reactive protein, thus directing removal of senescent and apoptotic cells or oxidized lipoproteins. In addition, OxPLs acquire novel biological activities not characteristic of their unoxidized precursors, including the ability to regulate innate and adaptive immune responses. Effects of OxPLs described in vitro and in vivo suggest their potential relevance in different pathologies, including atherosclerosis, acute inflammation, lung injury, and many other conditions. This review summarizes current knowledge on the mechanisms of formation, structures, and biological activities of OxPLs. Furthermore, potential applications of OxPLs as disease biomarkers, as well as experimental therapies targeting OxPLs, are described, providing a broad overview of an emerging class of lipid mediators.
Figures





References
-
- Ago T. Kitazono T. Ooboshi H. Iyama T. Han YH. Takada J. Wakisaka M. Ibayashi S. Utsumi H. Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. - PubMed
-
- Alcaraz MJ. Fernandez P. Guillen MI. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9:2541–2551. - PubMed
-
- Ameli S. Hultgardh-Nilsson A. Regnstrom J. Calara F. Yano J. Cercek B. Shah PK. Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. - PubMed
-
- Androulakis N. Durand H. Ninio E. Tsoukatos DC. Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J Lipid Res. 2005;46:1923–1932. - PubMed
-
- Anton R. Camacho M. Puig L. Vila L. Hepoxilin B3 and its enzymatically formed derivative trioxilin B3 are incorporated into phospholipids in psoriatic lesions. J Invest Dermatol. 2002;118:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

