Human embryonic stem cell-derived keratinocytes exhibit an epidermal transcription program and undergo epithelial morphogenesis in engineered tissue constructs
- PMID: 19686061
- PMCID: PMC2810995
- DOI: 10.1089/ten.TEA.2009.0325
Human embryonic stem cell-derived keratinocytes exhibit an epidermal transcription program and undergo epithelial morphogenesis in engineered tissue constructs
Abstract
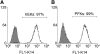
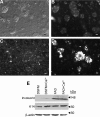
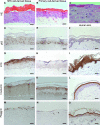
Human embryonic stem (hES) cells are an attractive source of cellular material for scientific, diagnostic, and potential therapeutic applications. Protocols are now available to direct hES cell differentiation to specific lineages at high purity under relatively defined conditions; however, researchers must establish the functional similarity of hES cell derivatives and associated primary cell types to validate their utility. Using retinoic acid to initiate differentiation, we generated high-purity populations of keratin 14+ (K14) hES cell-derived keratinocyte (hEK) progenitors and performed microarray analysis to compare the global transcriptional program of hEKs and primary foreskin keratinocytes. Transcriptional patterns were largely similar, though gene ontology analysis identified that genes associated with signal transduction and extracellular matrix were upregulated in hEKs. In addition, we evaluated the ability of hEKs to detect and respond to environmental stimuli such as Ca(2+), serum, and culture at the air-liquid interface. When cultivated on dermal constructs formed with collagen gels and human dermal fibroblasts, hEKs survived and proliferated for 3 weeks in engineered tissue constructs. Maintenance at the air-liquid interface induced stratification of surface epithelium, and immunohistochemistry results indicated that markers of differentiation (e.g., keratin 10, involucrin, and filaggrin) were localized to suprabasal layers. Although the overall tissue morphology was significantly different compared with human skin samples, organotypic cultures generated with hEKs and primary foreskin keratinocytes were quite similar, suggesting these cell types respond to this microenvironment in a similar manner. These results represent an important step in characterizing the functional similarity of hEKs to primary epithelia.
Figures
Similar articles
-
Effect of fibroblasts on epidermal regeneration.Br J Dermatol. 2002 Aug;147(2):230-43. doi: 10.1046/j.1365-2133.2002.04871.x. Br J Dermatol. 2002. PMID: 12174092
-
Mixture of fibroblasts and adipose tissue-derived stem cells can improve epidermal morphogenesis of tissue-engineered skin.Cells Tissues Organs. 2012;195(3):197-206. doi: 10.1159/000324921. Epub 2011 Apr 15. Cells Tissues Organs. 2012. PMID: 21494022
-
Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study.Lancet. 2009 Nov 21;374(9703):1745-53. doi: 10.1016/S0140-6736(09)61496-3. Lancet. 2009. PMID: 19932355
-
Regulation of epidermal stratification and development by basal keratinocytes.J Cell Physiol. 2023 Apr;238(4):742-748. doi: 10.1002/jcp.30978. Epub 2023 Feb 23. J Cell Physiol. 2023. PMID: 36815398 Review.
-
Decoding the Human Epidermal Complexity at Single-Cell Resolution.Int J Mol Sci. 2023 May 10;24(10):8544. doi: 10.3390/ijms24108544. Int J Mol Sci. 2023. PMID: 37239891 Free PMC article. Review.
Cited by
-
Engineering the human pluripotent stem cell microenvironment to direct cell fate.Biotechnol Adv. 2013 Nov 15;31(7):1002-19. doi: 10.1016/j.biotechadv.2013.03.002. Epub 2013 Mar 17. Biotechnol Adv. 2013. PMID: 23510904 Free PMC article. Review.
-
Efficient generation of functional epithelial and epidermal cells from human pluripotent stem cells under defined conditions.Tissue Eng Part C Methods. 2013 Dec;19(12):949-60. doi: 10.1089/ten.TEC.2013.0011. Epub 2013 Jun 4. Tissue Eng Part C Methods. 2013. PMID: 23560510 Free PMC article.
-
Which are the cells of origin in merkel cell carcinoma?J Skin Cancer. 2012;2012:680410. doi: 10.1155/2012/680410. Epub 2012 Dec 13. J Skin Cancer. 2012. PMID: 23304516 Free PMC article.
-
Directed differentiation of human pluripotent stem cells into epidermal keratinocyte-like cells.STAR Protoc. 2022 Aug 8;3(3):101613. doi: 10.1016/j.xpro.2022.101613. eCollection 2022 Sep 16. STAR Protoc. 2022. PMID: 35990735 Free PMC article.
-
Merkel Cell Polyomavirus: Oncogenesis in a Stable Genome.Viruses. 2021 Dec 30;14(1):58. doi: 10.3390/v14010058. Viruses. 2021. PMID: 35062263 Free PMC article. Review.
References
-
- Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. - PubMed
-
- D'Amour K.A. Bang A.G. Eliazer S. Kelly O.G. Agulnick A.D. Smart N.G. Moorman M.A. Kroon E. Carpenter M.K. Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392. - PubMed
-
- Nistor G.I. Totoiu M.O. Haque N. Carpenter M.K. Keirstead H.S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385. - PubMed
-
- Metallo C.M. Mohr J.C. Detzel C.J. de Pablo J.J. van Wie B.J. Palecek S.P. Engineering the stem cell microenvironment. Biotechnol Prog. 2007;23:18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

