Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors
- PMID: 19686589
- PMCID: PMC2741438
- DOI: 10.1186/1749-8104-4-32
Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors
Abstract
Background: The paired homeobox protein Pax6 is essential for proliferation and pluripotency of retinal progenitors. However, temporal changes in Pax6 protein expression associated with the generation of various retinal neurons have not been characterized with regard to the cell cycle. Here, we examine the dynamic changes of Pax6 expression among chicken retinal progenitors as they progress through the neurogenic cell cycle, and determine the effects of altered Pax6 levels on retinogenesis.
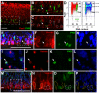
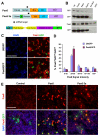
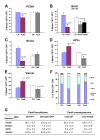
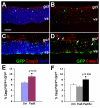
Results: We provide evidence that during the preneurogenic to neurogenic transition, Pax6 protein levels in proliferating progenitor cells are down-regulated. Neurogenic retinal progenitors retain a relatively low level of Pax6 protein, whereas postmitotic neurons either elevate or extinguish Pax6 expression in a cell type-specific manner. Cell imaging and cell cycle analyses show that neurogenic progenitors in the S phase of the cell cycle contain low levels of Pax6 protein, whereas a subset of progenitors exhibits divergent levels of Pax6 protein upon entering the G2 phase of the cell cycle. We also show that M phase cells contain varied levels of Pax6, and some correlate with the onset of early neuronal marker expression, forecasting cell cycle exit and cell fate commitment. Furthermore, either elevating or knocking down Pax6 attenuates cell proliferation and results in increased cell death. Reducing Pax6 decreases retinal ganglion cell genesis and enhances cone photoreceptor and amacrine interneuron production, whereas elevating Pax6 suppresses cone photoreceptor and amacrine cell fates.
Conclusion: These studies demonstrate for the first time quantitative changes in Pax6 protein expression during the preneurogenic to neurogenic transition and during the neurogenic cell cycle. The results indicate that Pax6 protein levels are stringently controlled in proliferating progenitors. Maintaining a relatively low Pax6 protein level is necessary for S phase re-entry, whereas rapid accumulation or reduction of Pax6 protein during the G2/M phase of the cell cycle may be required for specific neuronal fates. These findings thus provide novel insights on the dynamic regulation of Pax6 protein among neurogenic progenitors and the temporal frame of neuronal fate determination.
Figures

Similar articles
-
Proneural gene ash1 promotes amacrine cell production in the chick retina.Dev Neurobiol. 2009 Feb 1-15;69(2-3):88-104. doi: 10.1002/dneu.20693. Dev Neurobiol. 2009. PMID: 19067322 Free PMC article.
-
Trophic factors and neuronal interactions regulate the cell cycle and Pax6 expression in Müller stem cells.J Neurosci Res. 2008 May 15;86(7):1459-71. doi: 10.1002/jnr.21606. J Neurosci Res. 2008. PMID: 18189319
-
Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration.Exp Eye Res. 2008 Nov;87(5):433-44. doi: 10.1016/j.exer.2008.07.009. Epub 2008 Aug 5. Exp Eye Res. 2008. PMID: 18718467 Free PMC article.
-
Regulation of eye formation by the Rx and pax6 homeobox genes.Cell Mol Life Sci. 2000 Feb;57(2):186-94. doi: 10.1007/PL00000683. Cell Mol Life Sci. 2000. PMID: 10766016 Free PMC article. Review.
-
Radial glia cells in the developing human brain.Neuroscientist. 2008 Oct;14(5):459-73. doi: 10.1177/1073858407313512. Epub 2008 May 8. Neuroscientist. 2008. PMID: 18467668 Free PMC article. Review.
Cited by
-
Hedgehog-dependent E3-ligase Midline1 regulates ubiquitin-mediated proteasomal degradation of Pax6 during visual system development.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10103-8. doi: 10.1073/pnas.1600770113. Epub 2016 Aug 23. Proc Natl Acad Sci U S A. 2016. PMID: 27555585 Free PMC article.
-
Cytoskeleton proteins previously considered exclusive to ganglion cells are transiently expressed by all retinal neuronal precursors.BMC Dev Biol. 2011 Jul 22;11:46. doi: 10.1186/1471-213X-11-46. BMC Dev Biol. 2011. PMID: 21781303 Free PMC article.
-
Structural organization and expression pattern of the canine RPGRIP1 isoforms in retinal tissue.Invest Ophthalmol Vis Sci. 2011 May 6;52(6):2989-98. doi: 10.1167/iovs.10-6094. Invest Ophthalmol Vis Sci. 2011. PMID: 21282582 Free PMC article.
-
Suppression of PAX6 promotes cell proliferation and inhibits apoptosis in human retinoblastoma cells.Int J Mol Med. 2014 Aug;34(2):399-408. doi: 10.3892/ijmm.2014.1812. Epub 2014 Jun 17. Int J Mol Med. 2014. PMID: 24939714 Free PMC article.
-
Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells.PLoS One. 2013 Sep 20;8(9):e76489. doi: 10.1371/journal.pone.0076489. eCollection 2013. PLoS One. 2013. PMID: 24073291 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

