Functional grading of mineral and collagen in the attachment of tendon to bone
- PMID: 19686644
- PMCID: PMC2726319
- DOI: 10.1016/j.bpj.2009.05.043
Functional grading of mineral and collagen in the attachment of tendon to bone
Abstract
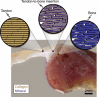
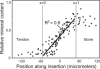
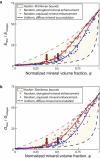
Attachment of dissimilar materials is a major challenge because high levels of localized stress may develop at their interfaces. An effective biologic solution to this problem exists at one of nature's most extreme interfaces: the attachment of tendon (a compliant, structural "soft tissue") to bone (a stiff, structural "hard tissue"). The goal of our study was to develop biomechanical models to describe how the tendon-to-bone insertion derives its mechanical properties. We examined the tendon-to-bone insertion and found two factors that give the tendon-to-bone transition a unique grading in mechanical properties: 1), a gradation in mineral concentration, measured by Raman spectroscopy; and 2), a gradation in collagen fiber orientation, measured by polarized light microscopy. Our measurements motivate a new physiological picture of the tissue that achieves this transition, the tendon-to-bone insertion, as a continuous, functionally graded material. Our biomechanical model suggests that the experimentally observed increase in mineral accumulation within collagen fibers can provide significant stiffening of the partially mineralized fibers, but only for concentrations of mineral above a "percolation threshold" corresponding to formation of a mechanically continuous mineral network within each collagen fiber (e.g., the case of mineral connectivity extending from one end of the fiber to the other). Increasing dispersion in the orientation distribution of collagen fibers from tendon to bone is a second major determinant of tissue stiffness. The combination of these two factors may explain the nonmonotonic variation of stiffness over the length of the tendon-to-bone insertion reported previously. Our models explain how tendon-to-bone attachment is achieved through a functionally graded material composition, and provide targets for tissue engineered surgical interventions and biomimetic material interfaces.
Figures



References
-
- Bostrom M.P.G., Boskey A., Kauffman J.K., Einhorn T.A. Form and function of bone. In: Buckwalter J.A., Einhorn T.A., Simon S.R., editors. Orthopaedic Basic Science. 2nd ed. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. pp. 319–370.
-
- Woo S.L., An K., Frank C.B., Livesay G.A., Ma C.B. Anatomy, biology, and biomechanics of tendon and ligament. In: Buckwalter J.A., Einhorn T.A., Simon S.R., editors. Orthopaedic Basic Science. 2nd ed. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. pp. 581–616.
-
- Szabo B.A., Babuska I. Wiley; New York: 1991. Finite Element Analysis.
-
- Benjamin M., Kumai T., Milz S., Boszczyk B.M., Boszczyk A.A. The skeletal attachment of tendons–tendon “entheses”. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;133:931–945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

