Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis
- PMID: 19686679
- PMCID: PMC2747264
- DOI: 10.1016/j.devcel.2009.06.017
Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis
Abstract
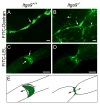
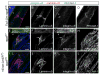
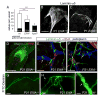
Dysfunction of lymphatic valves underlies human lymphedema, yet the process of valve morphogenesis is poorly understood. Here, we show that during embryogenesis, lymphatic valve leaflet formation is initiated by upregulation of integrin-alpha9 expression and deposition of its ligand fibronectin-EIIIA (FN-EIIIA) in the extracellular matrix. Endothelial cell-specific deletion of Itga9 (encoding integrin-alpha9) in mouse embryos results in the development of rudimentary valve leaflets characterized by disorganized FN matrix, short cusps, and retrograde lymphatic flow. Similar morphological and functional defects are observed in mice lacking the EIIIA domain of FN. Mechanistically, we demonstrate that in primary human lymphatic endothelial cells, the integrin-alpha9-EIIIA interaction directly regulates FN fibril assembly, which is essential for the formation of the extracellular matrix core of valve leaflets. Our findings reveal an important role for integrin-alpha9 signaling during lymphatic valve morphogenesis and implicate it as a candidate gene for primary lymphedema caused by valve defects.
Figures




References
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

