Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis
- PMID: 19686681
- PMCID: PMC2762206
- DOI: 10.1016/j.devcel.2009.07.013
Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis
Abstract
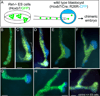
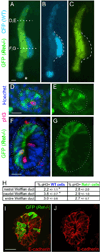
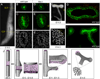
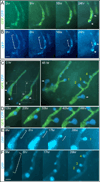
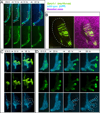
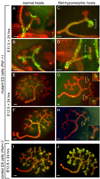
While the genetic control of renal branching morphogenesis has been extensively described, the cellular basis of this process remains obscure. GDNF/RET signaling is required for ureter and kidney development, and cells lacking Ret are excluded from the tips of the branching ureteric bud in chimeric kidneys. Here, we find that this exclusion results from earlier Ret-dependent cell rearrangements in the caudal Wolffian duct, which generate a specialized epithelial domain that later emerges as the tip of the primary ureteric bud. By juxtaposing cells with elevated or reduced RET activity, we find that Wolffian duct cells compete, based on RET signaling levels, to contribute to this domain. At the same time, the caudal Wolffian duct transiently converts from a simple to a pseudostratified epithelium, a process that does not require Ret. Thus, both Ret-dependent cell movements and Ret-independent changes in the Wolffian duct epithelium contribute to ureteric bud formation.
Figures

References
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. - PubMed
-
- Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–2064. - PubMed
-
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. - PubMed
-
- Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. - PubMed
-
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

