Chemical modification of class II G protein-coupled receptor ligands: frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies
- PMID: 19686775
- PMCID: PMC2815023
- DOI: 10.1016/j.pharmthera.2009.07.006
Chemical modification of class II G protein-coupled receptor ligands: frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies
Abstract
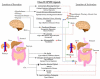
Recent research and clinical data have begun to demonstrate the huge potential therapeutic importance of ligands that modulate the activity of the secretin-like, Class II, G protein-coupled receptors (GPCRs). Ligands that can modulate the activity of these Class II GPCRs may have important clinical roles in the treatment of a wide variety of conditions such as osteoporosis, diabetes, amyotrophic lateral sclerosis and autism spectrum disorders. While these receptors present important new therapeutic targets, the large glycoprotein nature of their cognate ligands poses many problems with respect to therapeutic peptidergic drug design. These native peptides often exhibit poor bioavailability, metabolic instability, poor receptor selectivity and resultant low potencies in vivo. Recently, increased attention has been paid to the structural modification of these peptides to enhance their therapeutic efficacy. Successful modification strategies have included d-amino acid substitutions, selective truncation, and fatty acid acylation of the peptide. Through these and other processes, these novel peptide ligand analogs can demonstrate enhanced receptor subtype selectivity, directed signal transduction pathway activation, resistance to proteolytic degradation, and improved systemic bioavailability. In the future, it is likely, through additional modification strategies such as addition of circulation-stabilizing transferrin moieties, that the therapeutic pharmacopeia of drugs targeted towards Class II secretin-like receptors may rival that of the Class I rhodopsin-like receptors that currently provide the majority of clinically used GPCR-based therapeutics. Currently, Class II-based drugs include synthesized analogs of vasoactive intestinal peptide for type 2 diabetes or parathyroid hormone for osteoporosis.
Figures
Similar articles
-
Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors.Acta Pharmacol Sin. 2012 Mar;33(3):300-11. doi: 10.1038/aps.2011.170. Epub 2012 Jan 23. Acta Pharmacol Sin. 2012. PMID: 22266723 Free PMC article. Review.
-
The family B1 GPCR: structural aspects and interaction with accessory proteins.Curr Drug Targets. 2012 Jan;13(1):103-15. doi: 10.2174/138945012798868434. Curr Drug Targets. 2012. PMID: 21777182 Review.
-
Class B1 GPCRs: insights into multireceptor pharmacology for the treatment of metabolic disease.Am J Physiol Endocrinol Metab. 2024 Nov 1;327(5):E600-E615. doi: 10.1152/ajpendo.00371.2023. Epub 2024 Jul 10. Am J Physiol Endocrinol Metab. 2024. PMID: 38984948 Free PMC article. Review.
-
Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors.Curr Pharm Des. 2010;16(28):3071-88. doi: 10.2174/138161210793292474. Curr Pharm Des. 2010. PMID: 20687879 Free PMC article. Review.
-
Rational development of a high-affinity secretin receptor antagonist.Biochem Pharmacol. 2020 Jul;177:113929. doi: 10.1016/j.bcp.2020.113929. Epub 2020 Mar 23. Biochem Pharmacol. 2020. PMID: 32217097 Free PMC article.
Cited by
-
Metabolic and hormonal signatures in pre-manifest and manifest Huntington's disease patients.Front Physiol. 2014 Jun 23;5:231. doi: 10.3389/fphys.2014.00231. eCollection 2014. Front Physiol. 2014. PMID: 25002850 Free PMC article.
-
The Protective Role of PAC1-Receptor Agonist Maxadilan in BCCAO-Induced Retinal Degeneration.J Mol Neurosci. 2016 Oct;60(2):186-94. doi: 10.1007/s12031-016-0818-4. Epub 2016 Aug 26. J Mol Neurosci. 2016. PMID: 27566170
-
Neuropeptides: keeping the balance between pathogen immunity and immune tolerance.Curr Opin Pharmacol. 2010 Aug;10(4):473-81. doi: 10.1016/j.coph.2010.03.003. Curr Opin Pharmacol. 2010. PMID: 20399708 Free PMC article. Review.
-
PACAP Inhibits β-cell Mass Expansion in a Mouse Model of Type II Diabetes: Persistent Suppressive Effects on Islet Density.Front Endocrinol (Lausanne). 2013 Mar 11;4:27. doi: 10.3389/fendo.2013.00027. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23483824 Free PMC article.
-
Cerebellum Transcriptome of Mice Bred for High Voluntary Activity Offers Insights into Locomotor Control and Reward-Dependent Behaviors.PLoS One. 2016 Nov 28;11(11):e0167095. doi: 10.1371/journal.pone.0167095. eCollection 2016. PLoS One. 2016. PMID: 27893846 Free PMC article.
References
-
- Aaboe K, Krarup T, Madsbad S, Holst J. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab. 2008;10:994–1003. - PubMed
-
- Aiyar N, Daines RA, Disa J, Chambers PA, Sauermelch CF, Quiniou M, et al. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J Pharmacol Exp Ther. 2001;296:768–775. - PubMed
-
- Alba M, Schally A, Salvatori R. Partial reversibility of growth hormone (GH) deficiency in the GH-releasing hormone (GHRH) knockout mouse by postnatal treatment with a GHRH analog. Endocrinology. 2005;146:1506–1513. - PubMed
-
- Amara S, Jonas V, Rosenfeld M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. - PubMed
-
- Arborelius L, Owens M, Plotsky P, Nemeroff C. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

