Resilience against predator stress and dendritic morphology of amygdala neurons
- PMID: 19686780
- PMCID: PMC4022315
- DOI: 10.1016/j.bbr.2009.08.014
Resilience against predator stress and dendritic morphology of amygdala neurons
Abstract
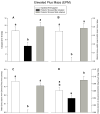
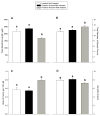
Individual differences in coping response lie at the core of vulnerability to conditions like post-traumatic stress disorder (PTSD). Like humans, not all animals exposed to severe stress show lasting change in affect. Predator stress is a traumatic experience inducing long-lasting fear, but not in all rodents. Thus, individual variation may be a cross species factor driving responsiveness to stressful events. The present study investigated neurobiological bases of variation in coping with severe stress. The amygdala was studied because it modulates fear and its function is affected by stress. Moreover, stress-induced plasticity of the amygdala has been related to induction of anxiety, a comorbid symptom of psychiatric conditions like PTSD. We exposed rodents to predator stress and grouped them according to their adaptability based on a standard anxiety test (the elevated plus maze). Subsequently we investigated if well-adapted (less anxious) and mal-adapted (extremely anxious) stressed animals differed in the structure of dendritic trees of their output neurons of the right basolateral amygdala (BLA). Two weeks after exposure to stress, well-adapted animals showed low anxiety levels comparable to unstressed controls, whereas mal-adapted animals were highly anxious. In these same animals, Golgi analysis revealed that BLA neurons of well-adapted rats exhibited more densely packed and shorter dendrites than neurons of mal-adapted or unstressed control animals, which did not differ. These data suggest that dendritic hypotrophy in the BLA may be a resilience marker against lasting anxiogenic effects of predator stress.
Figures

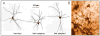


References
-
- Adamec R. Does Long Term Potentiation in Periacqueductal Gray (PAG) Mediate Lasting Changes in Rodent ALB Produced by Predator Stress? -Effects of Low Frequency Stimulation (LFS) of PAG on Place Preference and Changes in ALB Produced by Predator Stress. Behavioural Brain Research. 2001;120:111–135. - PubMed
-
- Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiology & Behavior. 2005;86(1-2):75–91. - PubMed
-
- Adamec R, Blundell J, Strasser K, Burton P. Mechanisms of lasting change in anxiety induced by severe stress. In: Sato N, Pitman R, editors. PTSD: Brain Mechanisms and Clinical Implications. Tokyo: Springer-Verlag; 2006. pp. 61–81. - PubMed
-
- Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals -- implications for understanding and treating affective disorder following traumatic stress in humans. Neuroscience & Biobehavioral Reviews. 1998;23(2):301–318. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

