Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial
- PMID: 19687005
- PMCID: PMC2762855
- DOI: 10.2196/jmir.1252
Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial
Abstract
Background: Telemonitoring of patients with chronic heart failure (CHF) is an emerging concept to detect early warning signs of impending acute decompensation in order to prevent hospitalization.
Objective: The goal of the MOBIle TELemonitoring in Heart Failure Patients Study (MOBITEL) was to evaluate the impact of home-based telemonitoring using Internet and mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation.
Methods: Patients were randomly allocated to pharmacological treatment (control group) or to pharmacological treatment with telemedical surveillance for 6 months (tele group). Patients randomized into the tele group were equipped with mobile phone-based patient terminals for data acquisition and data transmission to the monitoring center. Study physicians had continuous access to the data via a secure Web portal. If transmitted values went outside individually adjustable borders, study physicians were sent an email alert. Primary endpoint was hospitalization for worsening CHF or death from cardiovascular cause.
Results: The study was stopped after randomization of 120 patients (85 male, 35 female); median age was 66 years (IQR 62-72). The control group comprised 54 patients (39 male, 15 female) with a median age of 67 years (IQR 61-72), and the tele group included 54 patients (40 male, 14 female) with a median age of 65 years (IQR 62-72). There was no significant difference between groups with regard to baseline characteristics. Twelve tele group patients were unable to begin data transmission due to the inability of these patients to properly operate the mobile phone ("never beginners"). Four patients did not finish the study due to personal reasons. Intention-to-treat analysis at study end indicated that 18 control group patients (33%) reached the primary endpoint (1 death, 17 hospitalizations), compared with 11 tele group patients (17%, 0 deaths, 11 hospitalizations; relative risk reduction 50%, 95% CI 3-74%, P = .06). Per-protocol analysis revealed that 15% of tele group patients (0 deaths, 8 hospitalizations) reached the primary endpoint (relative risk reduction 54%, 95% CI 7-79%, P= .04). NYHA class improved by one class in tele group patients only (P< .001). Tele group patients who were hospitalized for worsening heart failure during the study had a significantly shorter length of stay (median 6.5 days, IQR 5.5-8.3) compared with control group patients (median 10.0 days, IQR 7.0-13.0; P= .04). The event rate of never beginners was not higher than the event rate of control group patients.
Conclusions: Telemonitoring using mobile phones as patient terminals has the potential to reduce frequency and duration of heart failure hospitalizations. Providing elderly patients with an adequate user interface for daily data acquisition remains a challenging component of such a concept.
Conflict of interest statement
None declared.
Figures


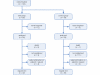
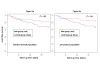
References
-
- Solomon Scott D, Dobson Joanna, Pocock Stuart, Skali Hicham, McMurray John J V, Granger Christopher B, Yusuf Salim, Swedberg Karl, Young James B, Michelson Eric L, Pfeffer Marc A. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007 Sep 25;116(13):1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=17724259CIRCULATIONAHA.107.696906 - DOI - PubMed
-
- Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Chronic Heart Failure, authors. ACC/AHA 2005 guidelines for the diagnosis and management of chronic heart failure in the adult. J Am Coll Cardiol. 2005;46(6):1144–1178. doi: 10.1016/j.jacc.2005.07.012.S0735-1097(05)01582-2 - DOI - PubMed
-
- Goldberg Lee R, Piette John D, Walsh Mary Norine, Frank Theodore A, Jaski Brian E, Smith Andrew L, Rodriguez Raymond, Mancini Donna M, Hopton Laurie A, Orav E John, Loh Evan. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: The Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003 Oct;146(4):705–712. doi: 10.1016/S0002-8703(03)00393-4.S0002870303003934 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

