Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection
- PMID: 19687200
- PMCID: PMC2772533
- DOI: 10.1128/IAI.00420-09
Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection
Abstract
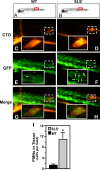
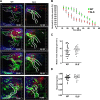
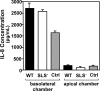
In contrast to infection of superficial tissues, Streptococcus pyogenes infection of deeper tissue can be associated with a significantly diminished inflammatory response, suggesting that this bacterium has the ability to both promote and suppress inflammation. To examine this, we analyzed the behavior of an S. pyogenes mutant deficient in expression of the cytolytic toxin streptolysin S (SLS-) and evaluated events that occur during the first few hours of infection by using several models including injection of zebrafish (adults, larvae, and embryos), a transepithelial polymorphonuclear leukocyte (PMN) migration assay, and two-photon microscopy of mice in vivo. In contrast to wild-type S. pyogenes, the SLS- mutant was associated with the robust recruitment of neutrophils and significantly reduced lethal myositis in adult zebrafish. Similarly, the mutant was attenuated in embryos in its ability to cause lethality. Infection of larva muscle allowed an analysis of inflammation in real time, which revealed that the mutant had recruited PMNs to the infection site. Analysis of transepithelial migration in vitro suggested that SLS inhibited the host cells' production of signals chemotactic for neutrophils, which contrasted with the proinflammatory effect of an unrelated cytolytic toxin, streptolysin O. Using two-photon microscopy of mice in vivo, we showed that the extravasation of neutrophils during infection with SLS- mutant bacteria was significantly accelerated compared to infection with wild-type S. pyogenes. Taken together, these data support a role for SLS in the inhibition of neutrophil recruitment during the early stages of S. pyogenes infection.
Figures




References
-
- Bakleh, M., L. E. Wold, J. N. Mandrekar, W. S. Harmsen, H. H. Dimashkieh, and L. M. Baddour. 2005. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin. Infect. Dis. 40:410-414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

