PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae
- PMID: 19687204
- PMCID: PMC2747926
- DOI: 10.1128/IAI.00486-09
PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae
Abstract
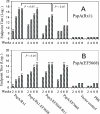
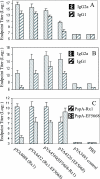
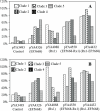
Pneumococcal surface protein A (PspA) is highly immunogenic and can induce a protective immune response against pneumococcal infection. PspA is divided into two major families based on serological variability: family 1 and family 2. To provide broad protection, PspA proteins from pneumococcal strains Rx1 (family 1) and EF5668 (family 2) were combined to form two PspA fusion proteins, PspA/Rx1-EF5668 and PspA/EF5668-Rx1. Each protein was fused to a type II secretion signal and delivered by a recombinant attenuated Salmonella vaccine (RASV). Both PspA/Rx1-EF5668 and PspA/EF5668-Rx1 were synthesized in the RASV and secreted into the periplasm and supernatant. The fusion proteins reacted strongly with both anti-PspA/Rx1 and anti-PspA/EF5668 antisera. Oral immunization of BALB/c mice with RASV synthesizing either PspA fusion protein elicited serum immunoglobulin G (IgG) and mucosal IgA responses against both families of PspA. Analysis of IgG isotypes (IgG2a and IgG1) indicated a strong Th1 bias to the immune responses to both proteins. Sera from mice immunized with RASV synthesizing PspA/Rx1-EF5668 bound to the surface and directed C3 complement deposition on representative strains from all five PspA clades. Immunization with RASV synthesizing either protein protected mice against intraperitoneal challenge with Streptococcus pneumoniae WU2 strain (family 1), intravenous challenge with S. pneumoniae 3JYP2670 strain (family 2), and intranasal challenge with S. pneumoniae A66.1 (family 1). The PspA/Rx1-EF5668 protein elicited significantly greater protection than PspA/EF5668-Rx1, PspA/Rx1, or PspA/EF5668. These results indicate an RASV synthesizing a PspA fusion protein representing both PspA families constitutes an effective antipneumococcal vaccine, extending and enhancing protection against multiple strains of S. pneumoniae.
Figures
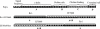


References
-
- Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. - PMC - PubMed
-
- Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. - PMC - PubMed
-
- Basset, A., C. M. Thompson, S. K. Hollingshead, D. E. Briles, E. W. Ades, M. Lipsitch, and R. Malley. 2007. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect. Immun. 75:5460-5464. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

