Steric and not structure-specific factors dictate the endocytic mechanism of glycosylphosphatidylinositol-anchored proteins
- PMID: 19687251
- PMCID: PMC2733760
- DOI: 10.1083/jcb.200903102
Steric and not structure-specific factors dictate the endocytic mechanism of glycosylphosphatidylinositol-anchored proteins
Abstract
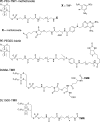
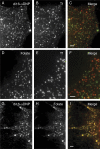
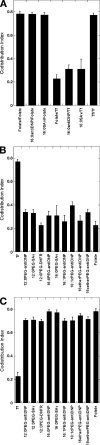
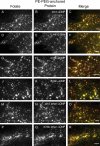
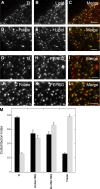
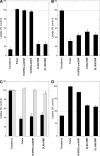
Diverse glycosylphosphatidylinositol (GPI)-anchored proteins enter mammalian cells via the clathrin- and dynamin-independent, Arf1-regulated GPI-enriched early endosomal compartment/clathrin-independent carrier endocytic pathway. To characterize the determinants of GPI protein targeting to this pathway, we have used fluorescence microscopic analyses to compare the internalization of artificial lipid-anchored proteins, endogenous membrane proteins, and membrane lipid markers in Chinese hamster ovary cells. Soluble proteins, anchored to cell-inserted saturated or unsaturated phosphatidylethanolamine (PE)-polyethyleneglycols (PEGs), closely resemble the GPI-anchored folate receptor but differ markedly from the transferrin receptor, membrane lipid markers, and even protein-free PE-PEGs, both in their distribution in peripheral endocytic vesicles and in the manner in which their endocytic uptake responds to manipulations of cellular Arf1 or dynamin activity. These findings suggest that the distinctive endocytic targeting of GPI proteins requires neither biospecific recognition of their GPI anchors nor affinity for ordered-lipid microdomains but is determined by a more fundamental property, the steric bulk of the lipid-anchored protein.
Figures


Comment in
-
Endocytosis of lipid-anchored proteins: excluding GEECs from the crowd.J Cell Biol. 2009 Aug 24;186(4):457-9. doi: 10.1083/jcb.200907119. Epub 2009 Aug 17. J Cell Biol. 2009. PMID: 19687254 Free PMC article. Review.
References
-
- Barlos K., Gatos D., Hatzi O., Koch N., Koutsogianni S. 1996. Synthesis of the very acid-sensitive Fmoc-Cys(Mmt)-OH and its application in solid-phase peptide synthesis.Int. J. Pept. Protein Res. 47:148–153 - PubMed
-
- Bonazzi M., Spanò S., Turacchio G., Cericola C., Valente C., Colanzi A., Kweon H.S., Hsu V.W., Polishchuck E.V., Polishchuck R.S., et al. 2005. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways.Nat. Cell Biol. 7:570–580 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

