Pancreatic proteolytic enzyme therapy compared with gemcitabine-based chemotherapy for the treatment of pancreatic cancer
- PMID: 19687327
- PMCID: PMC2860407
- DOI: 10.1200/JCO.2009.22.8429
Pancreatic proteolytic enzyme therapy compared with gemcitabine-based chemotherapy for the treatment of pancreatic cancer
Abstract
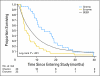
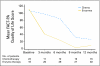
PURPOSE Conventional medicine has had little to offer patients with inoperable pancreatic adenocarcinoma; thus, many patients seek alternative treatments. The National Cancer Institute, in 1998, sponsored a randomized, phase III, controlled trial of proteolytic enzyme therapy versus chemotherapy. Because most eligible patients refused random assignment, the trial was changed in 2001 to a controlled, observational study. METHODS All patients were seen by one of the investigators at Columbia University, and patients who received enzyme therapy were seen by the participating alternative practitioner. Of 55 patients who had inoperable pancreatic cancer, 23 elected gemcitabine-based chemotherapy, and 32 elected enzyme treatment, which included pancreatic enzymes, nutritional supplements, detoxification, and an organic diet. Primary and secondary outcomes were overall survival and quality of life, respectively. Results At enrollment, the treatment groups had no statistically significant differences in patient characteristics, pathology, quality of life, or clinically meaningful laboratory values. Kaplan-Meier analysis found a 9.7-month difference in median survival between the chemotherapy group (median survival, 14 months) and enzyme treatment groups (median survival, 4.3 months) and found an adjusted-mortality hazard ratio of the enzyme group compared with the chemotherapy group of 6.96 (P < .001). At 1 year, 56% of chemotherapy-group patients were alive, and 16% of enzyme-therapy patients were alive. The quality of life ratings were better in the chemotherapy group than in the enzyme-treated group (P < .01). CONCLUSION Among patients who have pancreatic cancer, those who chose gemcitabine-based chemotherapy survived more than three times as long (14.0 v 4.3 months) and had better quality of life than those who chose proteolytic enzyme treatment.
Trial registration: ClinicalTrials.gov NCT00003851.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Conventional and complementary therapies: a tale of two research standards?J Clin Oncol. 2010 Apr 20;28(12):1979-81. doi: 10.1200/JCO.2010.28.5320. Epub 2010 Mar 22. J Clin Oncol. 2010. PMID: 20308650 No abstract available.
References
-
- Ries L, Malbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review, 1975-2005 (of November 2007 SEER data submission) http://seer.cancer.gov/csr/1975_2005/
-
- Gonzalez NJ. Enzyme Therapy and Cancer. http://www.dr-gonzalez.com/history_of_treatment.htm.
-
- Gonzalez NJ, Isaaacs LL. Evaluation of pancreatic proteolytic enzyme treatment of adenocarcinoma of the pancreas, with nutrition and detoxification support. Nutr Cancer. 1999;33:117–124. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Burris HA., 3rd Recent updates on the role of chemotherapy in pancreatic cancer. Semin Oncol. 2005;32(suppl 6):S1–S3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

