Hippocampus leads ventral striatum in replay of place-reward information
- PMID: 19688032
- PMCID: PMC2717326
- DOI: 10.1371/journal.pbio.1000173
Hippocampus leads ventral striatum in replay of place-reward information
Abstract
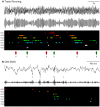
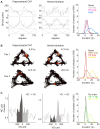
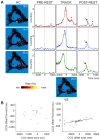
Associating spatial locations with rewards is fundamental to survival in natural environments and requires the integrity of the hippocampus and ventral striatum. In joint multineuron recordings from these areas, hippocampal-striatal ensembles reactivated together during sleep. This process was especially strong in pairs in which the hippocampal cell processed spatial information and ventral striatal firing correlated to reward. Replay was dominated by cell pairs in which the hippocampal "place" cell fired preferentially before the striatal reward-related neuron. Our results suggest a plausible mechanism for consolidating place-reward associations and are consistent with a central tenet of consolidation theory, showing that the hippocampus leads reactivation in a projection area.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples.J Neurosci. 2004 Jul 21;24(29):6446-56. doi: 10.1523/JNEUROSCI.0575-04.2004. J Neurosci. 2004. PMID: 15269254 Free PMC article.
-
Reward cues in space: commonalities and differences in neural coding by hippocampal and ventral striatal ensembles.J Neurosci. 2012 Sep 5;32(36):12444-59. doi: 10.1523/JNEUROSCI.0593-12.2012. J Neurosci. 2012. PMID: 22956836 Free PMC article.
-
Theta phase precession in rat ventral striatum links place and reward information.J Neurosci. 2011 Feb 23;31(8):2843-54. doi: 10.1523/JNEUROSCI.4869-10.2011. J Neurosci. 2011. PMID: 21414906 Free PMC article.
-
Reactivation in ventral striatum during hippocampal ripples: evidence for the binding of reward and spatial memories?J Neurosci. 2008 Oct 1;28(40):9895-7. doi: 10.1523/JNEUROSCI.3778-08.2008. J Neurosci. 2008. PMID: 18829947 Free PMC article. Review. No abstract available.
-
Learning, memory and consolidation mechanisms for behavioral control in hierarchically organized cortico-basal ganglia systems.Hippocampus. 2020 Jan;30(1):73-98. doi: 10.1002/hipo.23167. Epub 2019 Oct 16. Hippocampus. 2020. PMID: 31617622 Free PMC article. Review.
Cited by
-
Imagination as a fundamental function of the hippocampus.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 19;377(1866):20210336. doi: 10.1098/rstb.2021.0336. Epub 2022 Oct 31. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36314152 Free PMC article. Review.
-
Active Reward Processing during Human Sleep: Insights from Sleep-Related Eating Disorder.Front Neurol. 2012 Nov 27;3:168. doi: 10.3389/fneur.2012.00168. eCollection 2012. Front Neurol. 2012. PMID: 23205019 Free PMC article.
-
The balance of forward and backward hippocampal sequences shifts across behavioral states.Hippocampus. 2013 Jan;23(1):22-9. doi: 10.1002/hipo.22049. Epub 2012 Jun 27. Hippocampus. 2013. PMID: 22736562 Free PMC article.
-
Persistence of hippocampal and striatal multivoxel patterns during awake rest after motor sequence learning.iScience. 2022 Nov 4;25(12):105498. doi: 10.1016/j.isci.2022.105498. eCollection 2022 Dec 22. iScience. 2022. PMID: 36404923 Free PMC article.
-
Coupling between slow waves and sharp-wave ripples engages distributed neural activity during sleep in humans.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2012075118. doi: 10.1073/pnas.2012075118. Proc Natl Acad Sci U S A. 2021. PMID: 34001599 Free PMC article.
References
-
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. - PubMed
-
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford (United Kingdom): Clarendon Press; 1978. 570
-
- Barnes C. A, McNaughton B. L, Mizumori S. J, Leonard B. W, Lin L. H. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. - PubMed
-
- Morris R. G, Garrud P, Rawlins J. N, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. - PubMed
-
- Kim J. J, Fanselow M. S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

