Persistent cAMP-signals triggered by internalized G-protein-coupled receptors
- PMID: 19688034
- PMCID: PMC2718703
- DOI: 10.1371/journal.pbio.1000172
Persistent cAMP-signals triggered by internalized G-protein-coupled receptors
Abstract
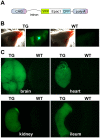
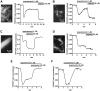
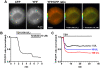
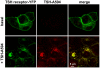
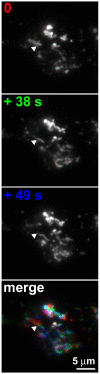
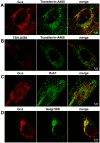
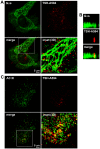
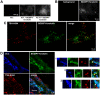
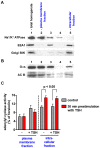
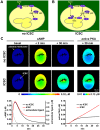
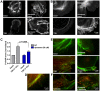
G-protein-coupled receptors (GPCRs) are generally thought to signal to second messengers like cyclic AMP (cAMP) from the cell surface and to become internalized upon repeated or prolonged stimulation. Once internalized, they are supposed to stop signaling to second messengers but may trigger nonclassical signals such as mitogen-activated protein kinase (MAPK) activation. Here, we show that a GPCR continues to stimulate cAMP production in a sustained manner after internalization. We generated transgenic mice with ubiquitous expression of a fluorescent sensor for cAMP and studied cAMP responses to thyroid-stimulating hormone (TSH) in native, 3-D thyroid follicles isolated from these mice. TSH stimulation caused internalization of the TSH receptors into a pre-Golgi compartment in close association with G-protein alpha(s)-subunits and adenylyl cyclase III. Receptors internalized together with TSH and produced downstream cellular responses that were distinct from those triggered by cell surface receptors. These data suggest that classical paradigms of GPCR signaling may need revision, as they indicate that cAMP signaling by GPCRs may occur both at the cell surface and from intracellular sites, but with different consequences for the cell.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Pierce K. L, Premont R. T, Lefkowitz R. J. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Pippig S, Andexinger S, Lohse M. J. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–676. - PubMed
-
- Sorkin A, von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. - PubMed
-
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. - PubMed
-
- Polo S, Di Fiore P. P. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

