Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni
- PMID: 19688043
- PMCID: PMC2721635
- DOI: 10.1371/journal.pntd.0000504
Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni
Abstract
Background: The near exclusive use of praziquantel (PZQ) for treatment of human schistosomiasis has raised concerns about the possible emergence of drug-resistant schistosomes.
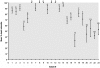
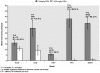
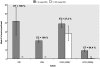
Methodology/principal findings: We measured susceptibility to PZQ of isolates of Schistosoma mansoni obtained from patients from Kisumu, Kenya continuously exposed to infection as a consequence of their occupations as car washers or sand harvesters. We used a) an in vitro assay with miracidia, b) an in vivo assay targeting adult worms in mice and c) an in vitro assay targeting adult schistosomes perfused from mice. In the miracidia assay, in which miracidia from human patients were exposed to PZQ in vitro, reduced susceptibility was associated with previous treatment of the patient with PZQ. One isolate ("KCW") that was less susceptible to PZQ and had been derived from a patient who had never fully cured despite multiple treatments was studied further. In an in vivo assay of adult worms, the KCW isolate was significantly less susceptible to PZQ than two other isolates from natural infections in Kenya and two lab-reared strains of S. mansoni. The in vitro adult assay, based on measuring length changes of adults following exposure to and recovery from PZQ, confirmed that the KCW isolate was less susceptible to PZQ than the other isolates tested. A sub-isolate of KCW maintained separately and tested after three years was susceptible to PZQ, indicative that the trait of reduced sensitivity could be lost if selection was not maintained.
Conclusions/significance: Isolates of S. mansoni from some patients in Kisumu have lower susceptibility to PZQ, including one from a patient who was never fully cured after repeated rounds of treatment administered over several years. As use of PZQ continues, continued selection for worms with diminished susceptibility is possible, and the probability of emergence of resistance will increase as large reservoirs of untreated worms diminish. The potential for rapid emergence of resistance should be an important consideration of treatment programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. - PubMed
-
- Fenwick A, Savioli L, Engels D, Bergquist RN, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–515. - PubMed
-
- Crompton DW. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397–403. - PubMed
-
- Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuenté LA. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. - PubMed
-
- Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr Opin Infect Dis. 2006;19:577–582. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

