Gemcitabine versus gemcitabine combined with cisplatin treatment locally advanced or metastatic pancreatic cancer: a retrospective analysis
- PMID: 19688061
- PMCID: PMC2699081
- DOI: 10.4143/crt.2008.40.1.22
Gemcitabine versus gemcitabine combined with cisplatin treatment locally advanced or metastatic pancreatic cancer: a retrospective analysis
Abstract
Purpose: Gemcitabine is the most active agent to treat unresectable pancreatic cancer. The superiority of combining other drugs with cisplatin is still controversial; therefore, we performed a retrospective analysis of gemcitabine versus gemcitabine combined with cisplatin to determine the treatment outcomes for patients with locally advanced or metastatic pancreatic cancer.
Materials and methods: From 2001 to 2007, we enrolled 60 patients who were treated with gemcitabine or gemcitabine combined with cisplatin for locally advanced or metastatic pancreatic cancer. Gemcitabine 1, 000 mg/m(2) (G) was administrated at day 1 and day 8 every 3 weeks. Cisplatin 60 mg/m(2) was added at day 1 every 3 weeks to the gemcitabine schedule (GP).
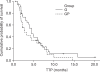
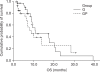
Results: NUMBER OF G: GP was 34: 26, locally advanced to metastatic ratio was 35% to 65% in group G and 46% to 54% in group GP. Median follow up duration was 29 months. The median number of chemotherapy cycles was 4 (range: 2 approximately 11) for the G group, and 4 (range: 1 approximately 11) for the GP group. The response rate of the G and GP groups was 17% and 11%, respectively. The progression free survival (PFS) was 4.5 months and 2.8 months, respectively, for the G and GP groups. The overall survival (OS) was 10.7 and 8.7 months respectively, for the G and GP groups, but there is no statistically significant difference of the PFS (p=0.2396) and OS (p=0.4643) between the 2 groups. The hematological toxicity profile was similar (the grade III neutropenia and thrombocytopenia was 4.4% and 3.1%, respectively, in G group, and 7.5% and 2.8%, respectively, in the GP group). But non-hematological toxicities such as skin rash, abnormal liver function and nausea/vomiting were observed in 3 patients of the GP group. On the prognostic factor analysis, no factors predicted a longer PFS and OS for both the G and GP groups.
Conclusions: Gemcitabine single treatment might be more tolerable and it had the same efficacy compared to cisplatin combination treatment in this retrospective study.
Keywords: Cisplatin; Gemcitabine; Pancreatic neoplasm.
Figures
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994;81:882–885. - PubMed
-
- Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. - PubMed
-
- Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA, 3rd, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–353. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials