Chlamydia trachomatis alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in HeLa-229 cells
- PMID: 19688112
- PMCID: PMC2727623
- DOI: 10.1155/2009/342032
Chlamydia trachomatis alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in HeLa-229 cells
Abstract
Chlamydia trachomatis (CT) is the leading cause of diseases related to reproductive health and iron plays important role in chlamydial pathogenesis. Iron homeostasis in chlamydia-infected cells is not clear thus far. This study shows that expression of the transferrin receptor (TfR) is downregulated, whereas expression of the ferritin heavy chain is upregulated in CT-infected HeLa-229 cells. Expression of iron-regulatory protein (IRP)-1 predominates over IRP-2 in infected cells. In infected cells, attenuated binding activity of IRP-iron responsive elements (IREs) is observed using the electrophoretic mobility-shift assay. These results suggest that iron homeostasis is modulated in CT-infected HeLa cells at the interface of acquisition and commensal use of iron.
Figures

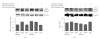

Similar articles
-
Iron regulatory protein 1 outcompetes iron regulatory protein 2 in regulating cellular iron homeostasis in response to nitric oxide.J Biol Chem. 2011 Jul 1;286(26):22846-54. doi: 10.1074/jbc.M111.231902. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566147 Free PMC article.
-
Ferritin heavy chain-mediated iron homoeostasis regulates expression of IL-10 in Chlamydia trachomatis-infected HeLa cells.Cell Biol Int. 2011 Aug;35(8):793-8. doi: 10.1042/CBI20100463. Cell Biol Int. 2011. PMID: 21413929
-
Excess capacity of the iron regulatory protein system.J Biol Chem. 2007 Aug 24;282(34):24650-9. doi: 10.1074/jbc.M703167200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17604281
-
Mammalian iron metabolism and its control by iron regulatory proteins.Biochim Biophys Acta. 2012 Sep;1823(9):1468-83. doi: 10.1016/j.bbamcr.2012.05.010. Epub 2012 May 17. Biochim Biophys Acta. 2012. PMID: 22610083 Free PMC article. Review.
-
Role of nitric oxide in cellular iron metabolism.Biometals. 2003 Mar;16(1):125-35. doi: 10.1023/a:1020788603046. Biometals. 2003. PMID: 12572672 Review.
Cited by
-
Strategies of Intracellular Pathogens for Obtaining Iron from the Environment.Biomed Res Int. 2015;2015:476534. doi: 10.1155/2015/476534. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26120582 Free PMC article. Review.
-
Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells.Autophagy. 2016 May 3;12(5):850-63. doi: 10.1080/15548627.2016.1160176. Autophagy. 2016. PMID: 27002654 Free PMC article.
-
The effect of bacterial challenge on ferritin regulation in the yellow fever mosquito, Aedes aegypti.Insect Sci. 2013 Oct;20(5):601-19. doi: 10.1111/j.1744-7917.2012.01581.x. Epub 2012 Dec 11. Insect Sci. 2013. PMID: 23956079 Free PMC article.
-
Genomewide Transcriptional Responses of Iron-Starved Chlamydia trachomatis Reveal Prioritization of Metabolic Precursor Synthesis over Protein Translation.mSystems. 2018 Feb 13;3(1):e00184-17. doi: 10.1128/mSystems.00184-17. eCollection 2018 Jan-Feb. mSystems. 2018. PMID: 29468197 Free PMC article.
-
Neisseria gonorrhoeae Limits Chlamydia trachomatis Inclusion Development and Infectivity in a Novel In Vitro Co-Infection Model.Front Cell Infect Microbiol. 2022 Jul 7;12:911818. doi: 10.3389/fcimb.2022.911818. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35873141 Free PMC article.
References
-
- Brunham RC, Binns B, Guijon F, et al. Etiology and outcome of acute pelvic inflammatory disease. Journal of Infectious Diseases. 1988;158(3):510–517. - PubMed
-
- Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends in Microbiology. 1997;5(7):288–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

