Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort
- PMID: 19689234
- PMCID: PMC2764863
- DOI: 10.2174/156720509788929273
Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort
Abstract
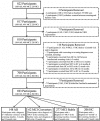
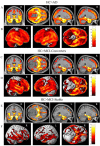
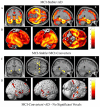
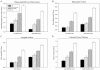
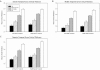
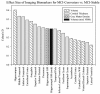
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multi-center study assessing neuroimaging in diagnosis and longitudinal monitoring. Amnestic Mild Cognitive Impairment (MCI) often represents a prodromal form of dementia, conferring a 10-15% annual risk of converting to probable AD. We analyzed baseline 1.5T MRI scans in 693 participants from the ADNI cohort divided into four groups by baseline diagnosis and one year MCI to probable AD conversion status to identify neuroimaging phenotypes associated with MCI and AD and potential predictive markers of imminent conversion. MP-RAGE scans were analyzed using publicly available voxel-based morphometry (VBM) and automated parcellation methods. Measures included global and hippocampal grey matter (GM) density, hippocampal and amygdalar volumes, and cortical thickness values from entorhinal cortex and other temporal and parietal lobe regions. The overall pattern of structural MRI changes in MCI (n=339) and AD (n=148) compared to healthy controls (HC, n=206) was similar to prior findings in smaller samples. MCI-Converters (n=62) demonstrated a very similar pattern of atrophic changes to the AD group up to a year before meeting clinical criteria for AD. Finally, a comparison of effect sizes for contrasts between the MCI-Converters and MCI-Stable (n=277) groups on MRI metrics indicated that degree of neurodegeneration of medial temporal structures was the best antecedent MRI marker of imminent conversion, with decreased hippocampal volume (left > right) being the most robust. Validation of imaging biomarkers is important as they can help enrich clinical trials of disease modifying agents by identifying individuals at highest risk for progression to AD.
Figures
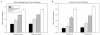
References
-
- Wimo A, Winblad B, Aguero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17:63–67. - PubMed
-
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. - PubMed
-
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
