CX3CR1-deficiency is associated with increased severity of disease in experimental autoimmune uveitis
- PMID: 19689733
- PMCID: PMC2747136
- DOI: 10.1111/j.1365-2567.2009.03046.x
CX3CR1-deficiency is associated with increased severity of disease in experimental autoimmune uveitis
Abstract
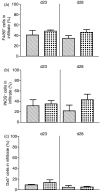
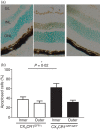
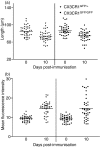
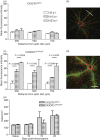
The role of CX3CR1 in regulating the function of monocytes and microglia was examined in mice in which CX3CR1 had been replaced by green fluorescent protein (GFP). Induction of experimental autoimmune uveitis (EAU) in these mice resulted in increased disease severity at day 23 postimmunization with uveitogenic peptide when compared with CX3CR1-positive mice and increased apoptosis of neuronal cells in the inner nuclear layer. Resident microglia within the retina were activated equally as EAU developed in mice with or without CX3CR1, as determined by changes in morphology, suggesting that the microglial cell response did not account for the differences. Although the inflammatory infiltrate had increased in mice without CX3CR1 at day 23 postimmunization, the percentage of natural killer cells in the infiltrate was not changed in these mice. Similarly, increased disease severity at this stage was not associated with an overall increased percentage of macrophages in the retinal inflammatory infiltrate or in increased activation of these cells. The increased recruitment of monocytes to the retina in response to EAU induction in CX3CR1(GFP/GFP) mice compared with CX3CR1(GFP/+) mice was not reflected in increased migration away from vessels, leading to marked clustering of GFP(+) cells around veins and venules in these mice. It is possible that this monocyte/macrophage clustering leads to the increased severity of disease seen in the mice by focusing and so intensifying the inflammatory response.
Figures

References
-
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–13. - PubMed
-
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. - PubMed
-
- Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. - PubMed
-
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

