Switch from perforin-expressing to perforin-deficient CD8(+) T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo
- PMID: 19689737
- PMCID: PMC2747140
- DOI: 10.1111/j.1365-2567.2009.03072.x
Switch from perforin-expressing to perforin-deficient CD8(+) T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo
Abstract
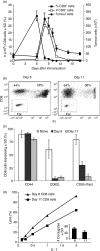
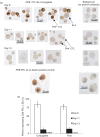
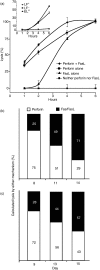
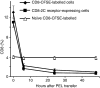
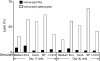
Although CD8(+) cytotoxic T lymphocytes (CTL) exhibit both Fas ligand (FasL) -based and perforin-based lytic activities, the accepted hallmark of a fully active CTL remains its perforin killing machinery. Yet the origin, rationale for possessing both a slow-acting (FasL) and a fast-acting (perforin) killing mechanism has remained enigmatic. Here we have investigated perforin expression in CTL directly involved in acute tumour (i.e. leukaemias EL4 and L1210) allograft rejection occurring within the peritoneal cavity. We show that at the height of the immune response, the majority of conjugate-forming CD8(+) CTL express high levels of perforin messenger RNA and protein, and kill essentially via perforin. Later however, coinciding with complete rejection, fully cytocidal CTL emerge which exhibit a stark decrease in perforin and now kill preferentially via constitutively expressed FasL. Although late in emergence, and persistent, these powerful CTL are neither effector-memory nor memory CTL. This finding has implications for the monitoring of anti-transplant responses in clinical settings, based on assessing perforin expression in graft infiltrating CD8(+) T cells. The results show that as the immune response progresses in vivo, targeted cellular suicide mainly prunes high perforin-expressing CD8(+) cells, resulting in the gradual switch in effector CTL, from mostly perforin-based to largely Fas/FasL-based killers. Hence, two kinds of CD8(+) CTL have two killing strategies.
Figures




References
-
- Cerottini JC, Brunner KT. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. - PubMed
-
- Kägi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–32. - PubMed
-
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. - PubMed
-
- Henkart PA, Catalfamo M. CD8+ effector cells. Adv Immunol. 2004;83:233–52. - PubMed
-
- Berke G, Clark W. Killer lymphocytes. Berlin: Springer; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

