Ribosomal protein S19 and S24 insufficiency cause distinct cell cycle defects in Diamond-Blackfan anemia
- PMID: 19689926
- PMCID: PMC2759502
- DOI: 10.1016/j.bbadis.2009.08.002
Ribosomal protein S19 and S24 insufficiency cause distinct cell cycle defects in Diamond-Blackfan anemia
Abstract
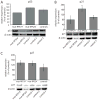
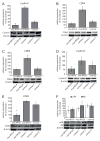
Diamond-Blackfan anemia (DBA) is a severe congenital anemia characterized by a specific decrease of erythroid precursors. The disease is also associated with growth retardation, congenital malformations, a predisposition for malignant disease and heterozygous mutations in either of the ribosomal protein (RP) genes RPS7, RPS17, RPS19, RPS24, RPL5, RPL11 and RPL35a. We show herein that primary fibroblasts from DBA patients with truncating mutations in RPS19 or in RPS24 have a marked reduction in proliferative capacity. Mutant fibroblasts are associated with extended cell cycles and normal levels of p53 when compared to w.t. cells. RPS19 mutant fibroblasts accumulate in the G1 phase, whereas the RPS24 mutant cells show an altered progression in the S phase resulting in reduced levels in the G2/M phase. RPS19 deficient cells exhibit reduced levels of Cyclin-E, CDK2 and retinoblastoma (Rb) protein supporting a cell cycle arrest in the G1 phase. In contrast, RPS24 deficient cells show increased levels of the cell cycle inhibitor p21 and a seemingly opposing increase in Cyclin-E, CDK4 and CDK6. In combination, our results show that RPS19 and RPS24 insufficient fibroblasts have an impaired growth caused by distinct blockages in the cell cycle. We suggest this proliferative constraint to be an important contributing mechanism for the complex extra-hematological features observed in DBA.
Figures



References
-
- Young NS, Alter B. Inherited bone marrow failure syndromes: Introduction. In: Young, Alter, editors. Aplastic anemia acquired and inherited. WB saunders Campany; Philadelphia, PA: 1994.
-
- Halperin DS, Freedman MH. Diamond-blackfan anemia: etiology, pathophysiology, and treatment. Am J Pediatr Hematol Oncol. 1989;11:380–394. - PubMed
-
- Willig TN, Draptchinskaia N, Dianzani I, Ball S, Niemeyer C, Ramenghi U, Orfali K, Gustavsson P, Garelli E, Brusco A, Tiemann C, Perignon JL, Bouchier C, Cicchiello L, Dahl N, Mohandas N, Tchernia G. Mutations in ribosomal protein S19 gene and diamond blackfan anemia: wide variations in phenotypic expression. Blood. 1999;94:4294–4306. - PubMed
-
- Gripp KW, McDonald-McGinn DM, La Rossa D, McGain D, Federman N, Vlachos A, Glader BE, McKenzie SE, Lipton JM, Zackai EH. Bilateral microtia and cleft palate in cousins with Diamond-Blackfan anemia. Am J Med Genet. 2001;101:268–274. - PubMed
-
- Gazda HT, Sheen MR, Vlachos A, Choesmel V, O’Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Zaucha JM, Glader B, Niemeyer C, Meerpohl JJ, Atsidaftos E, Lipton JM, Gleizes PE, Beggs AH. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet. 2008;83:769–780. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

