Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages
- PMID: 19690049
- PMCID: PMC2736863
- DOI: 10.1242/jcs.053207
Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages
Abstract
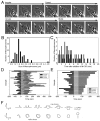
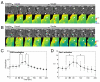
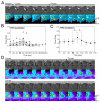
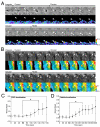
Macropinosomes are large endocytic vesicles that form in ruffling regions of plasma membrane. To analyze signal organization relative to ruffle closure into circular ruffles and cup closure into macropinosomes, this study used quantitative microscopy to measure 3' phosphoinositides and small-GTPase activities in a representative subset of forming macropinosomes. Macropinocytosis was stimulated by the addition of macrophage colony-stimulating factor (M-CSF) to macrophages expressing fluorescent reporter proteins. Ratiometric and fluorescence resonance energy transfer (FRET) microscopy determined that Rac1 activity and phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P(3)] levels increased transiently, peaking 26-30 seconds after ruffle closure. Three-dimensional reconstruction of cells labeled with the fluorescent dye FM4-64 showed that PtdIns(3,4,5)P(3) was restricted to open, circular cups in the plasma membrane. Quantitative fluorescence microscopic methods determined the timing of cup closure, which followed 40-100 seconds after Rac1 and PtdIns(3,4,5)P(3) deactivation and coincided with accumulation of phosphatidylinositol 3-phosphate and Rab5a. Thus, ruffle closure creates a circular domain of plasma membrane that localizes the activation and deactivation of Rac1 and phosphoinositide 3-kinase (PI3K), followed by recruitment of Rab5a and the contractile activities of cup closure.
Figures



References
-
- Amyere, M., Payrastre, B., Krause, U., van der Smissen, P., Veithen, A. and Courtoy, P. J. (2000). Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 11, 3453-3467. - PMC - PubMed
-
- Araki, N., Hatae, T., Furukawa, A. and Swanson, J. A. (2003). Phosphoinositide-3-kinase-independent contractile activites associated with Fcγ-receptor-mediated phagocytosis and macropinocytosis in macrophages. J. Cell Sci. 116, 247-257. - PubMed
-
- Araki, N., Hamasaki, M., Egami, Y. and Hatae, T. (2006). Effect of 3-methylamide on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Struct. Funct. 31, 145-157. - PubMed
-
- Araki, N., Egami, Y., Watanabe, Y. and Hatae, T. (2007). Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 313, 1496-1507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

