Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients
- PMID: 19690143
- PMCID: PMC2770839
- DOI: 10.1158/0008-5472.CAN-09-0347
Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients
Abstract
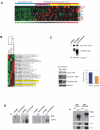
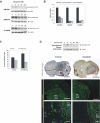
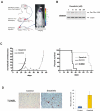
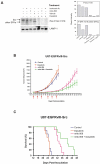
Activating epidermal growth factor receptor (EGFR) mutations are common in many cancers including glioblastoma. However, clinical responses to EGFR inhibitors are infrequent and short-lived. We show that the Src family kinases (SFK) Fyn and Src are effectors of oncogenic EGFR signaling, enhancing invasion and tumor cell survival in vivo. Expression of a constitutively active EGFR mutant, EGFRvIII, resulted in activating phosphorylation and physical association with Src and Fyn, promoting tumor growth and motility. Gene silencing of Fyn and Src limited EGFR- and EGFRvIII-dependent tumor cell motility. The SFK inhibitor dasatinib inhibited invasion, promoted tumor regression, and induced apoptosis in vivo, significantly prolonging survival of an orthotopic glioblastoma model expressing endogenous EGFRvIII. Dasatinib enhanced the efficacy of an anti-EGFR monoclonal antibody (mAb 806) in vivo, further limiting tumor growth and extending survival. Examination of a large cohort of clinical samples showed frequent coactivation of EGFR and SFKs in glioblastoma patients. These results establish a mechanism linking EGFR signaling with Fyn and Src activation to promote tumor progression and invasion in vivo and provide rationale for combined anti-EGFR and anti-SFK targeted therapies.
Figures


References
-
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. - PubMed
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. - PubMed
-
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

