Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation
- PMID: 19690147
- PMCID: PMC2762204
- DOI: 10.1158/0008-5472.CAN-08-4447
Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation
Abstract
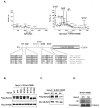
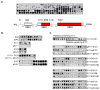
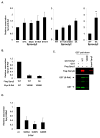
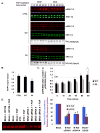
Sprouty2 is a feedback regulator that controls the Ras/Raf/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase (MAPK) pathway at multiple levels, one way being through direct interaction with Raf kinases. Consistent with a role as a tumor suppressor, Sprouty2 expression is often down-regulated in human cancers. However, Sprouty2 is up-regulated in some cancers, suggesting the existence of posttranscriptional mechanisms that permit evasion of Sprouty2-mediated antitumorigenic properties. We report that MAPK activation induces Sprouty2 phosphorylation on six serine residues, which reduced Sprouty2 association with wild-type B-Raf. Mutation of these six serines to nonphosphorylatable alanines increased the ability of Sprouty2 to inhibit growth factor-induced MAPK activation. Oncogenic B-Raf mutants such as B-Raf V600E did not associate with Sprouty2, but this resistance to Sprouty2 binding was not due to phosphorylation. Instead, the active kinase conformation induced by oncogenic mutation prevents Sprouty2 binding. These results reveal a dual mechanism that affects the Sprouty2/B-Raf interaction: Sprouty phosphorylation and B-Raf conformation.
Figures


References
-
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–63. - PubMed
-
- de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, Bellusci S. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev. 1999;81:213–6. - PubMed
-
- Tefft JD, Lee M, Smith S, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–22. - PubMed
-
- Minowada G, Jarvis LA, Chi CL, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75. - PubMed
-
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

