Thioflavin S (NSC71948) interferes with Bcl-2-associated athanogene (BAG-1)-mediated protein-protein interactions
- PMID: 19690191
- PMCID: PMC2775257
- DOI: 10.1124/jpet.109.153601
Thioflavin S (NSC71948) interferes with Bcl-2-associated athanogene (BAG-1)-mediated protein-protein interactions
Abstract
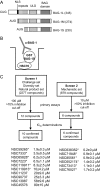
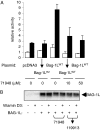
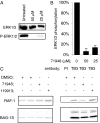
The C-terminal BAG domain is thought to play a key role in BAG-1-induced survival and proliferation by mediating protein-protein interactions, for example, with heat shock proteins HSC70 and HSP70, and with RAF-1 kinase. Here, we have identified thioflavin S (NSC71948) as a potential small-molecule chemical inhibitor of these interactions. NSC71948 inhibited the interaction of BAG-1 and HSC70 in vitro and decreased BAG-1:HSC70 and BAG-1:HSP70 binding in intact cells. NSC71948 also reduced binding between BAG-1 and RAF-1, but had no effect on the interaction between two unrelated proteins, BIM and MCL-1. NSC71948 functionally reversed the ability of BAG-1 to promote vitamin D3 receptor-mediated transactivation, an activity of BAG-1 that depends on HSC70/HSP70 binding, and reduced phosphorylation of p44/42 mitogen-activate protein kinase. NSC71948 can be used to stain amyloid fibrils; however, structurally related compounds, thioflavin T and BTA-1, had no effect on BAG-1:HSC70 binding, suggesting that structural features important for amyloid fibril binding and inhibition of BAG-1:HSC70 binding may be separable. We demonstrated that NSC71948 inhibited the growth of BAG-1 expressing human ZR-75-1 breast cancer cells and wild-type, but not BAG-1-deficient, mouse embryo fibroblasts. Taken together, these data suggest that NSC71948 may be a useful molecule to investigate the functional significance of BAG-1 C-terminal protein interactions. However, it is important to recognize that NSC71948 may exert additional "off-target" effects. Inhibition of BAG-1 function may be an attractive strategy to inhibit the growth of BAG-1-overexpressing cancers, and further screens of additional compound collections may be warranted.
Figures

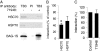

References
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Hohfeld J. (2002) Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem 277:45920–45927 - PubMed
-
- Briknarová K, Takayama S, Brive L, Havert ML, Knee DA, Velasco J, Homma S, Cabezas E, Stuart J, Hoyt DW, et al. (2001) Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat Struct Biol 8:349–352 - PubMed
-
- Clemo NK, Collard TJ, Southern SL, Edwards KD, Moorghen M, Packham G, Hague A, Paraskeva C, Williams AC. (2008) BAG-1 is up-regulated in colorectal tumour progression and promotes colorectal tumour cell survival through increased NF-kappaB activity. Carcinogenesis 29:849–857 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

