All of the human beta-type globin genes compete for LCR enhancer activity in embryonic erythroid cells of yeast artificial chromosome transgenic mice
- PMID: 19690216
- PMCID: PMC2812047
- DOI: 10.1096/fj.09-137778
All of the human beta-type globin genes compete for LCR enhancer activity in embryonic erythroid cells of yeast artificial chromosome transgenic mice
Abstract
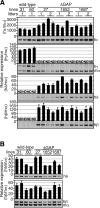
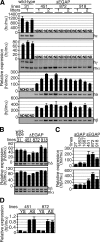
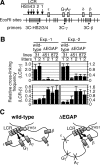
In primitive erythroid cells of human beta-globin locus transgenic mice (TgM), the locus control region (LCR)-proximal epsilon- and gamma-globin genes are transcribed, whereas the distal delta- and beta-globin genes are silent. It is generally accepted that the beta-globin gene is competitively suppressed by gamma-globin gene expression at this developmental stage. Previously, however, we observed that epsilon-globin gene expression was severely attenuated when its distance from the LCR was extended, implying that beta-globin gene might also be silenced because of its great distance from the LCR. Here, to clarify the beta-globin gene silencing mechanism, we established TgM lines carrying either gamma- or epsilon- plus gamma-globin promoter deletions, without significantly altering the distance between the beta-globin gene and the LCR. Precocious expression of delta- and beta-globin genes was observed in primitive erythroid cells of mutant, but not wild-type TgM, which was most evident when both the epsilon and gamma promoters were deleted. Thus, we clearly demonstrated that the repression of the delta- and beta-globin genes in primitive erythroid cells is dominated by competitive silencing by the epsilon- and gamma-globin gene promoters, and that epsilon- and the other beta-like globin genes might be activated by two distinct mechanisms by the LCR.
Figures

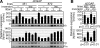
Similar articles
-
DNase I hypersensitivity and epsilon-globin transcriptional enhancement are separable in locus control region (LCR) HS1 mutant human beta-globin YAC transgenic mice.J Biol Chem. 2010 May 7;285(19):14495-503. doi: 10.1074/jbc.M110.116525. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231293 Free PMC article.
-
Linear distance from the locus control region determines epsilon-globin transcriptional activity.Mol Cell Biol. 2007 Aug;27(16):5664-72. doi: 10.1128/MCB.00602-07. Epub 2007 Jun 4. Mol Cell Biol. 2007. PMID: 17548470 Free PMC article.
-
Human beta-globin locus control region HS5 contains CTCF- and developmental stage-dependent enhancer-blocking activity in erythroid cells.Mol Cell Biol. 2003 Dec;23(24):8946-52. doi: 10.1128/MCB.23.24.8946-8952.2003. Mol Cell Biol. 2003. PMID: 14645507 Free PMC article.
-
beta-YAC transgenic mice for studying LCR function.Ann N Y Acad Sci. 1998 Jun 30;850:28-37. doi: 10.1111/j.1749-6632.1998.tb10459.x. Ann N Y Acad Sci. 1998. PMID: 9668524 Review.
-
Chromatin looping as a target for altering erythroid gene expression.Ann N Y Acad Sci. 2016 Mar;1368(1):31-9. doi: 10.1111/nyas.13012. Epub 2016 Feb 25. Ann N Y Acad Sci. 2016. PMID: 26918894 Free PMC article. Review.
Cited by
-
Disrupting the adult globin promoter alleviates promoter competition and reactivates fetal globin gene expression.Blood. 2022 Apr 7;139(14):2107-2118. doi: 10.1182/blood.2021014205. Blood. 2022. PMID: 35090172 Free PMC article.
-
Transcriptional environment and chromatin architecture interplay dictates globin expression patterns of heterospecific hybrids derived from undifferentiated human embryonic stem cells or from their erythroid progeny.Exp Hematol. 2013 Nov;41(11):967-979.e6. doi: 10.1016/j.exphem.2013.08.005. Epub 2013 Aug 28. Exp Hematol. 2013. PMID: 23993951 Free PMC article.
-
Epigenetic modifications and chromosome conformations of the beta globin locus throughout development.Stem Cell Rev Rep. 2013 Aug;9(4):397-407. doi: 10.1007/s12015-012-9355-x. Stem Cell Rev Rep. 2013. PMID: 22374078 Free PMC article.
-
Combined approaches for increasing fetal hemoglobin (HbF) and de novo production of adult hemoglobin (HbA) in erythroid cells from β-thalassemia patients: treatment with HbF inducers and CRISPR-Cas9 based genome editing.Front Genome Ed. 2023 Jul 17;5:1204536. doi: 10.3389/fgeed.2023.1204536. eCollection 2023. Front Genome Ed. 2023. PMID: 37529398 Free PMC article. Review.
References
-
- Stamatoyannopoulos G. Philadelphia/London: W. B. Saunders; The Molecular Basis of Blood Diseases. 1994
-
- Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. - PubMed
-
- Raich N, Enver T, Nakamoto B, Josephson B, Papayannopoulou T, Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990;250:1147–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

