Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene
- PMID: 19690465
- PMCID: PMC2837199
- DOI: 10.4161/chan.3.4.9381
Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene
Abstract
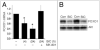
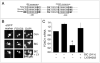
Activation of gene expression by FOXO transcription factors can promote neuronal death in response to loss of trophic support, or oxidative stress. The predominant neuronal FOXOs, FOXO1 and FOXO3, promote the expression of pro-death genes, such as Fas Ligand, Bim and Txnip. Neuroprotective signals initiated by neurotrophins, growth factors or synaptic activity trigger the nuclear export of FOXOs via activation of the PI3K-Akt pathway. One key aspect of FOXO regulation is that once PI3K-Akt activity has returned to baseline, FOXOs return to the nucleus to resume the activation of their target genes. Thus, the FOXO-inhibiting capacity of the PI3K-Akt pathway is thought to be short-lived. However, we show here that synaptic NMDA receptor activity not only triggers FOXO export, but also suppresses the expression of FOXO1. Blockade of PI3K activity prevents both FOXO nuclear export and suppression of FOXO1 expression, raising the possibility that FOXO1 is itself a FOXO target gene. We found that FOXO3, and to a lesser extent FOXO1 transactivates the FOXO1 promoter via a consensus FOXO binding site (GTA AAC AA), and also an upstream sequence resembling a classical FOXO-binding insulin response sequence (CAA AAC AA). Activity-dependent suppression of the FOXO1 promoter is mediated through the proximal GTAAACAA sequence. Similar suppression via this site is observed by activating neuronal IGF-1 receptors by exogenous insulin. Thus, through a feed-forward inhibition mechanism, synaptic activity triggers FOXO export resulting in suppression of FOXO1 expression. These results suggest that FOXO-inactivating signals are likely to result in longer-term inhibition of FOXO target gene expression than previously thought.
Figures

References
-
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. - PubMed
-
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. - PubMed
-
- Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–74. - PubMed
-
- Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci. 2005;1055:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
