Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2
- PMID: 19690545
- PMCID: PMC2743360
- DOI: 10.1038/sj.bjc.6605248
Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2
Abstract
Background: We have previously shown that cannabinoids induce growth inhibition and apoptosis in prostate cancer PC-3 cells, which express high levels of cannabinoid receptor types 1 and 2 (CB(1) and CB(2)). In this study, we investigated the role of CB(2) receptor in the anti-proliferative action of cannabinoids and the signal transduction triggered by receptor ligation.
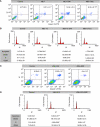
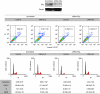
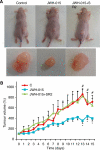
Methods: The human prostate cancer cell lines, namely PC-3, DU-145 and LNCaP, were used for this study. Cell proliferation was measured using MTT proliferation assay, [(3)H]-thymidine incorporation assay and cell-cycle study by flow cytometry. Ceramide quantification was performed using the DAG kinase method. The CB(2) receptor was silenced with specific small interfering RNA, and was blocked pharmacologically with SR 144528. In vivo studies were conducted by the induction of prostate xenograft tumours in nude mice.
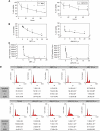
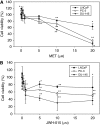
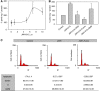
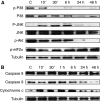
Results: We found that the anandamide analogue, R(+)-Methanandamide (MET), as well as JWH-015, a synthetic CB(2) agonist, exerted anti-proliferative effects in PC-3 cells. R(+)-Methanandamide- and JWH-015-induced cell death was rescued by treatment with the CB(2) receptor antagonist, SR 144528. Downregulation of CB(2) expression reversed the effects of JWH-015, confirming the involvement of CB(2) in the pro-apoptotic effect of cannabinoids. Further analysing the mechanism of JWH-015-induced cell growth inhibition, we found that JWH-015 triggered a de novo synthesis of ceramide, which was involved in cannabinoid-induced cell death, insofar as blocking ceramide synthesis with Fumonisin B1 reduced cell death. Signalling pathways activated by JWH-015 included JNK (c-Jun N-terminal kinase) activation and Akt inhibition. In vivo treatment with JWH-015 caused a significant reduction in tumour growth in mice.
Conclusions: This study defines the involvement of CB(2)-mediated signalling in the in vivo and in vitro growth inhibition of prostate cancer cells and suggests that CB(2) agonists have potential therapeutic interest and deserve to be explored in the management of prostate cancer.
Figures

References
-
- Ashton JC, Wright JL, McPartland JM, Tyndall JD (2008) Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr Med Chem 15: 1428–1443 - PubMed
-
- Bahnson R (2007) Androgen deprivation therapy for prostate cancer. J Urol 178: 1148. - PubMed
-
- Bifulco M, Malfitano AM, Pisanti S, Laezza C (2008) Endocannabinoids in endocrine and related tumours. Endocr Relat Cancer 15: 391–408 - PubMed
-
- Blazquez C, Salazar M, Carracedo A, Lorente M, Egia A, Gonzalez-Feria L, Haro A, Velasco G, Guzman M (2008) Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res 68: 1945–1952 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

