Ceruloplasmin (2-D PAGE) Pattern and Copper Content in Serum and Brain of Alzheimer Disease Patients
- PMID: 19690651
- PMCID: PMC2716788
Ceruloplasmin (2-D PAGE) Pattern and Copper Content in Serum and Brain of Alzheimer Disease Patients
Abstract
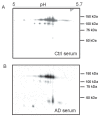
A dysfunction in copper homeostasis seems to occur in Alzheimer's disease (AD). We previously evidenced that an excess of non-ceruloplasmin-copper (NCC) correlated with the main functional, anatomical as well as cerebrospinal markers of the disease. Aim of our study was to investigate ceruloplasmin isoforms as potential actors in this AD copper dysfunction. Our data show that AD patients have ceruloplasmin fragments of low molecular weight (<50 kDa) both in their serum and brain, contrary to healthy controls. Ceruloplasmin isoforms of higher molecular weight (115 and 135 kDa in serum and 135 kDa in brain), as well as copper levels in the brain, instead, do not seem to mark a difference between AD and healthy subjects. These data suggest a ceruloplasmin fragmentation in the serum of AD patients. Some clues in this direction have been found also in the AD brain.
Keywords: Alzheimer’s disease; SDS-PAGE; brain; ceruloplasmin; copper; serum.
Figures


References
-
- Abe A, Yamashita S, Noma A. Sensitive, direct colorimetric assay for copper in serum. Clin Chem. 1989;35(4):552–4. - PubMed
-
- Barnham KJ, McKinstry WJ, Multhaup G, et al. Structure of the Alzheimer’s disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J Biol Chem. 2003;278:17401–7. - PubMed
-
- Bomboi G, Marchione F, Sepe-Monti M, et al. Correlation between metal ions and clinical findings in subjects affected by Alzheimer’s disease. Ann Ist Super Sanità. 2005;41:205–12. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
