High frequencies of exposure to the novel human parvovirus PARV4 in hemophiliacs and injection drug users, as detected by a serological assay for PARV4 antibodies
- PMID: 19691429
- PMCID: PMC2914696
- DOI: 10.1086/605646
High frequencies of exposure to the novel human parvovirus PARV4 in hemophiliacs and injection drug users, as detected by a serological assay for PARV4 antibodies
Abstract
Background: PARV4 is a human parvovirus that was first detected in and cloned from an individual with a human immunodeficiency virus (HIV) seroconversion-like illness and that subsequently persisted in the lymphoid tissue and bone marrow. In contrast to human parvovirus B19 infections, PARV4 infections are most frequently detected in injection drug users (IDUs), particularly those who are coinfected with HIV type 1 (HIV-1). To investigate the routes of transmission of PARV4 and to ascertain whether infections are acquired through plasma-derived blood products, we developed a novel anti-PARV4 enzyme-linked immunosorbent assay (ELISA) to determine its seroprevalence in subjects with parenteral exposure.
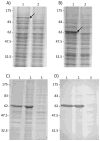
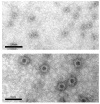
Methods: PARV4 viral protein 2 (VP2) was expressed and used as antigen in an indirect ELISA, to detect anti-PARV4 immunoglobulin G.
Results: All 50 adult control subjects who were nonparenterally exposed to PARV4 were anti-PARV4 negative, in contrast to HIV-infected and HIV-uninfected IDUs, who had antibody frequencies of 67% and 33%, respectively. Predominantly parenteral transmission was confirmed by the finding of similar frequencies of infection among HIV-coinfected and HIV-uninfected hemophiliacs (11 of 20 individuals and 4 of 15 individuals, respectively) who were treated with nonvirally inactivated factor VIII/factor IX, whereas all but 1 of the 35 nonhemophiliac siblings of these siblings were found to be seronegative (despite having close household contact).
Conclusions: The present study provides convincing evidence that PARV4 is primarily transmitted parenterally. Evidence for widespread infection of hemophiliacs treated with nonvirally inactivated clotting factor creates fresh safety concerns for plasma-derived blood products, particularly because parvoviruses are relatively resistant to virus inactivation.
Conflict of interest statement
Conflict of Interest: Eric Delwart and his institution (Blood Systems Research Institute and Department of Laboratory Medicine, University of California - San Francisco) have filed a patent describing the discovery of PARV4 and claim intellectual property rights. There are no actual or potential conflicts of interests for other authors.
Figures

References
-
- Lurcharchaiwong W, Chieochansin T, Payungporn S, Theamboonlers A, Poovorawan Y. Parvovirus 4 (PARV4) in serum of intravenous drug users and blood donors. Infection. 2008;36:488–91. - PubMed
-
- Soderlund-Venermo M, Hokynar K, Nieminen J, Rautakorpi H, Hedman K. Persistence of human parvovirus B19 in human tissues. Pathol Biol (Paris) 2002;50:307–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

