Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming 'vaccine resistant malaria'
- PMID: 19691559
- PMCID: PMC2730200
- DOI: 10.1111/j.1365-3024.2009.01138.x
Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming 'vaccine resistant malaria'
Abstract
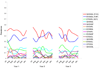
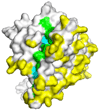
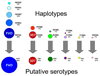
The development of effective malaria vaccines may be hindered by extensive genetic diversity in the surface proteins being employed as vaccine antigens. Understanding of the extent and dynamics of genetic diversity in vaccine antigens is needed to guide rational vaccine design and to interpret the results of vaccine efficacy trials conducted in malaria endemic areas. Molecular epidemiological, population genetic, and structural approaches are being employed to try to identify immunologically relevant polymorphism in vaccine antigens. The results of these studies will inform choices of which alleles to include in multivalent or chimeric vaccines; however, additional molecular and immuno-epidemiological studies in a variety of geographic locations will be necessary for these approaches to succeed. Alternative means of overcoming antigenic diversity are also being explored, including boosting responses to critical conserved regions of current vaccine antigens, identifying new, more conserved and less immunodominant antigens, and developing whole-organism vaccines. Continued creative application and integration of tools from multiple disciplines, including epidemiology, immunology, molecular biology, and evolutionary genetics and genomics, will likely be required to develop broadly protective vaccines against Plasmodium and other antigenically complex pathogens.
Figures
References
-
- Beall B, McEllistrem MC, Gertz RE, Jr, Wedel S, Boxrud DJ, Gonzalez AL, Medina MJ, Pai R, Thompson TA, Harrison LH, McGee L, Whitney CG. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol. 2006;44(3):999–1017. - PMC - PubMed
-
- Gonzalez BE, Hulten KG, Lamberth L, Kaplan SL, Mason EO., Jr Streptococcus pneumoniae serogroups 15 and 33: an increasing cause of pneumococcal infections in children in the United States after the introduction of the pneumococcal 7-valent conjugate vaccine. Pediatr Infect Dis J. 2006;25(4):301–305. - PubMed
-
- Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck HP, Alpers MP. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185(6):820–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

