Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles
- PMID: 19692128
- PMCID: PMC2761534
- DOI: 10.1016/j.jneuroim.2009.07.007
Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles
Abstract
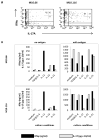
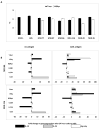
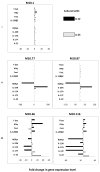
Th1/Th17 cells, secreting both IFNgamma and IL-17, are often associated with inflammatory pathology. We cloned and studied the cytokine phenotypes of MBP-specific, TCR-identical encephalitogenic CD4+ cells in relationship to Th1- and Th17-associated transcription factors T-bet and RORgammat. IFNgamma-producing cells could be sub-divided into those that are T-bet(+)/RORgammat(-) and those that are T-bet(+)/RORgammat(+). The latter comprises a spectrum of phenotypes, as defined by IL-17 production, and can be induced to up-regulate IL-23R with IL-12 or IL-23. The former, bona fide Th1 cells, lack IL-23R expression under all conditions. In vivo, T-bet(+)/RORgammat(-) and T-bet(+)/RORgammat(+) clones induce EAE equally well.
Figures
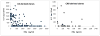




References
-
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

