Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR)
- PMID: 19692509
- PMCID: PMC2775392
- DOI: 10.1152/jn.00480.2009
Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR)
Erratum in
- J Neurophysiol. 2009 Dec;102(6):3781
Abstract
The mammalian brain is an enormously complex set of circuits composed of interconnected neuronal cell types. The analysis of central neural circuits will be greatly served by the ability to turn off specific neuronal cell types while recording from others in intact brains. Because drug delivery cannot be restricted to specific cell types, this can only be achieved by putting "silencer" transgenes under the control of neuron-specific promoters. Towards this end we have created a line of transgenic mice putting the Drosophila allatostatin (AL) neuropeptide receptor (AlstR) under the control of the tetO element, thus enabling its inducible expression when crossed to tet-transactivator lines. Mammals have no endogenous AL or AlstR, but activation of exogenously expressed AlstR in mammalian neurons leads to membrane hyperpolarization via endogenous G-protein-coupled inward rectifier K(+) channels, making the neurons much less likely to fire action potentials. Here we show that this tetO/AlstR line is capable of broadly expressing AlstR mRNA in principal neurons throughout the forebrain when crossed to a commercially-available transactivator line. We electrophysiologically characterize this cross in hippocampal slices, demonstrating that bath application of AL leads to hyperpolarization of CA1 pyramidal neurons, making them refractory to the induction of action potentials by injected current. Finally, we demonstrate the ability of AL application to silence the sound-evoked spiking responses of auditory cortical neurons in intact brains of AlstR/tetO transgenic mice. When crossed to other transactivator lines expressing in defined neuronal cell types, this AlstR/tetO line should prove a very useful tool for the analysis of intact central neural circuits.
Figures
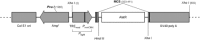
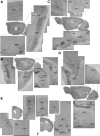

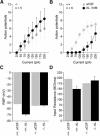
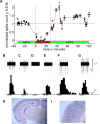
 , sound presentation. H and I: photomicrographs of AlstR mRNA in situ hybridization of a coronal slice from the brain of this mouse. Primary auditory cortex is located in the boxed region, which is expanded in I.
, sound presentation. H and I: photomicrographs of AlstR mRNA in situ hybridization of a coronal slice from the brain of this mouse. Primary auditory cortex is located in the boxed region, which is expanded in I.
References
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–156, 2007 - PubMed
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-time scale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005 - PubMed
-
- Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol 54: 495–503, 1998 - PubMed
-
- Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res 537: 333–336, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

