Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions
- PMID: 19692590
- PMCID: PMC2767377
- DOI: 10.1242/jcs.052704
Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions
Abstract
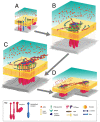
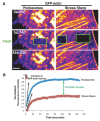
The invasiveness of cells is correlated with the presence of dynamic actin-rich membrane structures called invadopodia, which are membrane protrusions that are associated with localized polymerization of sub-membrane actin filaments. Similar to focal adhesions and podosomes, invadopodia are cell-matrix adhesion sites. Indeed, invadopodia share several features with podosomes, but whether they are distinct structures is still a matter of debate. Invadopodia are built upon an N-WASP-dependent branched actin network, and the Rho GTPase Cdc42 is involved in inducing invadopodial-membrane protrusion, which is mediated by actin filaments that are organized in bundles to form an actin core. Actin-core formation is thought to be an early step in invadopodium assembly, and the actin core is perpendicular to the extracellular matrix and the plasma membrane; this contrasts with the tangential orientation of actin stress fibers anchored to focal adhesions. In this Commentary, we attempt to summarize recent insights into the actin dynamics of invadopodia and podosomes, and the forces that are transmitted through these invasive structures. Although the mechanisms underlying force-dependent regulation of invadopodia and podosomes are largely unknown compared with those of focal adhesions, these structures do exhibit mechanosensitivity. Actin dynamics and associated forces might be key elements in discriminating between invadopodia, podosomes and focal adhesions. Targeting actin-regulatory molecules that specifically promote invadopodium formation is an attractive strategy against cancer-cell invasion.
Figures



References
-
- Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–16851. - PubMed
-
- Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, Kessler H, Spatz JP. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004;5:383–388. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

