The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats
- PMID: 19692603
- PMCID: PMC6665786
- DOI: 10.1523/JNEUROSCI.0505-09.2009
The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats
Abstract
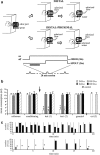
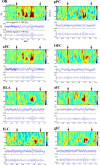
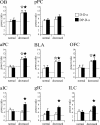
Recent findings have revealed the importance of orthonasal and retronasal olfaction in food memory, especially in conditioned odor aversion (COA); however, little is known about the dynamics of the cerebral circuit involved in the recognition of an odor as a toxic food signal and whether the activated network depends on the way (orthonasal vs retronasal) the odor was first experienced. In this study, we mapped the modulations of odor-induced oscillatory activities through COA learning using multisite recordings of local field potentials in behaving rats. During conditioning, orthonasal odor alone or associated with ingested odor was paired with immediate illness. For all animals, COA retrieval was assessed by orthonasal smelling only. Both types of conditioning induced similarly strong COA. Results pointed out (1) a predictive correlation between the emergence of powerful beta (15-40 Hz) activity and the behavioral expression of COA and (2) a differential network distribution of this beta activity according to the way the animals were exposed to the odor during conditioning. Indeed, for both types of conditioning, the aversive behavior was predicted by the emergence of a strong beta oscillatory activity in response to the odor in the olfactory bulb, piriform cortex, orbitofrontal cortex, and basolateral amygdala. This network was selectively extended to the infralimbic and insular cortices when the odor was ingested during acquisition. These differential networks could participate in different food odor memory; these results are discussed in line with recent behavioral results that indicate that COA can be formed over long odor-illness delays only if the odor is ingested.
Figures




References
-
- Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. - PubMed
-
- Bermúdez-Rattoni F, Grijalva CV, Kiefer SW, Garcia J. Flavor-illness aversions: the role of the amygdala in the acquisition of taste-potentiated odor aversions. Physiol Behav. 1986;38:503–508. - PubMed
-
- Boix-Trelis N, Vale-Martínez A, Guillazo-Blanch G, Martí-Nicolovius M. Muscarinic cholinergic receptor blockade in the rat prelimbic cortex impairs the social transmission of food preference. Neurobiol Learn Mem. 2007;87:659–668. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
