Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation
- PMID: 19692604
- PMCID: PMC3849831
- DOI: 10.1523/JNEUROSCI.2500-09.2009
Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation
Abstract
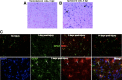
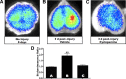
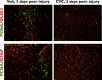
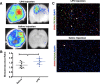
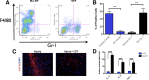
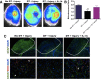
The adult mammalian brain responds to injury by activating a program of cell proliferation during which many oligodendrocyte precursors, microglia, and some astrocytes proliferate. Another common response to brain injury is the induction of reactive gliosis, a process whereby dormant astrocytes undergo morphological changes and alter their transcriptional profiles. Although brain injury-induced reactive gliosis is concurrent with the proliferation of surrounding cells, a functional relationship between reactive gliosis and this cell proliferation has not been clearly demonstrated. Here, we show that the mitogen sonic hedgehog (SHH) is produced in reactive astrocytes after injury to the cerebral cortex and participates in regulating the proliferation of Olig2-expressing (Olig2(+)) cells after brain injury. Using a cortical freeze injury to induce reactive gliosis in a Gli-luciferase reporter mouse, we show that the SHH pathway is maximally active 3 d after brain injury and returns to baseline levels by 14 d. SHH expression parallels Gli activation and localizes to glial fibrillary acidic protein-expressing reactive astrocytes. Inhibition of the SHH pathway with cyclopamine blocks the Gli response and significantly reduces both the proliferating and overall number of Olig2(+) cells in the injured cortex. To provide mechanistic insight into SHH pathway activation in astrocytes, we show that proinflammatory stimuli activate SHH-expressing reactive astrocytes, whereas inhibition of inflammation-induced reactive gliosis by macrophage depletion abolishes SHH activation after brain injury and dampens cell proliferation after injury. Our data describes a unique reactive astrocyte-based, SHH-expressing niche formed in response to injury and inflammation that regulates the proliferation of Olig2(+) cells.
Figures


References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
-
- Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, Blasberg RG, Holland EC. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–2249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
