Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms
- PMID: 19692613
- PMCID: PMC6665790
- DOI: 10.1523/JNEUROSCI.1978-09.2009
Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms
Abstract
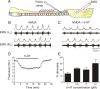
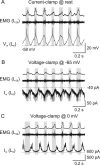
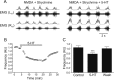
Serotonin (5-HT) plays an important role in shaping the activity of the spinal networks underlying locomotion in many vertebrate preparations. At larval stages in zebrafish, 5-HT does not change the frequency of spontaneous swimming; and it only decreases the quiescent period between consecutive swimming episodes. However, it is not known whether 5-HT exerts similar actions on the locomotor network at later developmental stages. For this, the effect of 5-HT on the fictive locomotor pattern of juvenile and adult zebrafish was analyzed. Bath-application of 5-HT (1-20 mum) reduced the frequency of the NMDA-induced locomotor rhythm. Blocking removal from the synaptic cleft with the reuptake inhibitor citalopram had similar effects, suggesting that endogenous serotonin is modulating the locomotor pattern. One target for this modulation was the mid-cycle inhibition during locomotion because the IPSPs recorded in spinal neurons during the hyperpolarized phase were increased both in amplitude and occurrence by 5-HT. Similar results were obtained for IPSCs recorded in spinal neurons clamped at the reversal potential of excitatory currents (0 mV). 5-HT also slows down the rising phase of the excitatory drive recorded in spinal cord neurons when glycinergic inhibition is blocked. These results suggest that the decrease in the locomotor burst frequency induced by 5-HT is mediated by a potentiation of mid-cycle inhibition combined with a delayed onset of the subsequent depolarization.
Figures






References
-
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. - PubMed
-
- Biró Z, Hill RH, Grillner S. 5-HT Modulation of identified segmental premotor interneurons in the lamprey spinal cord. J Neurophysiol. 2006;96:931–935. - PubMed
-
- Bracci E, Beato M, Nistri A. Extracellular K+ induces locomotor-like patterns in the rat spinal cord in vitro: comparison with NMDA or 5-HT induced activity. J Neurophysiol. 1998;79:2643–2652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
