Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells
- PMID: 19692632
- PMCID: PMC2803232
- DOI: 10.1194/jlr.M000729
Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells
Abstract
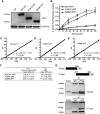
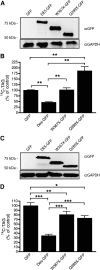
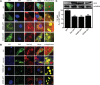
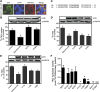
Murine desnutrin/human ATGL is a triacylglycerol (TAG) hydrolase with a predicted catalytic dyad within an alpha-beta hydrolase fold in the N-terminal region. In humans, mutations resulting in C-terminal truncation cause neutral lipid storage disease with myopathy. To identify critical functional domains, we measured TAG breakdown in cultured cells by mutated or truncated desnutrin. In vitro, C-terminally truncated desnutrin displayed an even higher apparent V(max) than the full-length form without changes in K(m), which may be explained by our finding of an interaction between the C- and N-terminal domains. In live cells, however, C-terminally truncated adenoviral desnutrin had lower TAG hydrolase activity. We investigated a role for the phosphorylation of C-terminal S406 and S430 residues but found that these were not necessary for TAG breakdown or lipid droplet localization in cells. The predicted N-terminal active sites, S47 and D166, were both critical for TAG hydrolysis in live cells and in vitro. We also identified two overlapping N-terminal motifs that predict lipid substrate binding domains, a glycine-rich motif (underlined) and an amphipathic alpha-helix (bold) within amino acid residues 10-24 (ISFAGCGFLGVYHIG). G14, F17, L18, and V20, but not G16 and G19, were important for TAG hydrolysis, suggesting a potential role for the amphipathic alpha-helix in TAG binding. This study identifies for the first time critical sites in the N-terminal region of desnutrin and reveals the requirement of the C-terminal region for TAG hydrolysis in cultured cells.
Figures

References
-
- Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. 2004. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975. - PubMed
-
- Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. 2004. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279: 47066–47075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

